

IM CANNABIS CORP.
ANNUAL INFORMATION FORM
For the Financial Year Ended December 31, 2020
April 26, 2021
TABLE OF CONTENTS
SCHEDULE "A" - AUDIT COMMITTEE CHARTER
-i-
ANNUAL INFORMATION FORM
In this annual information form ("Annual Information Form" or "AIF"), unless otherwise noted or the context indicates otherwise, the "Company" "IMCC", "we", "us" and "our" refer to IM Cannabis Corp., together with its subsidiaries, on a consolidated basis, and the "Group" refers to the Company, its subsidiaries, and Focus, an Israeli private company over which IMC Holdings exercises "de facto control" under IFRS 10. All dollar amounts referred to in this Annual Information Form are stated in Canadian dollars unless otherwise indicated. IMCC prepares its financial statements in accordance with IFRS as issued by the International Accounting Standards Board.
The information in this Annual Information Form is presented as at December 31, 2020 unless otherwise indicated. All references to the Company's Common Shares and securities issuable into Common Shares such as Warrants, Options, Broker Options, and RSUs are reflected on a post-Consolidation (as defined below) basis unless otherwise indicated or the context requires otherwise.
FORWARD-LOOKING STATEMENTS
Certain statements in this Annual Information Form may contain "forward-looking information" and "forward-looking statements" within the meaning of applicable securities laws (collectively referred to herein as "forward-looking statements"). All statements other than statements of fact may be deemed to be forward-looking statements, including statements with regard to expected financial performance, strategy and business conditions. The words "believe", "plan", "intend", "estimate", "expect", "anticipate", "continue", or "potential", and similar expressions, as well as future or conditional verbs such as "will", "should", "would", and "could" often identify forward-looking statements. These statements reflect management's current beliefs with respect to future events and are based on information currently available to management as of the date of this Annual Information Form including reasonable assumptions, estimates, internal and external analysis and opinions of management considering its experience, perception of trends, current conditions and expected developments as well as other factors that management believes to be relevant as at the date such statements are made.
Without limitation, this Annual Information Form contains forward-looking statements pertaining to:
- 2 -
With respect to the forward looking-statements contained in this Annual Information Form, the Company has made assumptions regarding, among other things:
Readers are cautioned that the above lists of forward-looking statements and assumptions are not exhaustive. Since forward-looking statements address future events and conditions, by their very nature they involve inherent risks and uncertainties. Actual results may differ materially from those currently anticipated or implied by such forward-looking statements due to a number of factors and risks. These include:
- 3 -
- 4 -
The foregoing list of risk factors is not exhaustive. Additional information on these and other factors that could affect the business, operations or financial results of the Company are detailed under the heading "Risk Factors" of this Annual Information Form. Unless otherwise indicated, forward-looking statements in this Annual Information Form describe our expectations as of the date of this Annual Information Form. The Company and management caution readers not to place undue reliance on any forward-looking statements, which speak only as of the date made. Although the Company believes that the expectations reflected in the forward-looking statements are reasonable, it can give no assurance that such expectations will prove to have been correct. The Company and management assume no obligation to update or revise them to reflect new events or circumstances except as required by applicable securities laws.
MARKET AND INDUSTRY DATA
This Annual Information Form contains market and industry data and forecasts obtained from third-party sources, industry publications and publicly available information. The Company believes that the industry data is accurate and that its estimates and assumptions are reasonable, but there is no assurance as to the accuracy or completeness of this data. Third-party sources generally state that the information contained therein has been obtained from sources believed to be reliable, but there can be no assurance as to the accuracy or completeness of included information. Although management believes it to be reliable, the Company has not independently verified any of the data from third-party sources referred to in this Annual Information Form, or analyzed or verified the underlying information relied upon or referred to by such sources, or ascertained the underlying economic assumptions relied upon by such sources.
NOTE REGARDING THE COMPANY'S ACCOUNTING PRACTICES
The Company complies with IFRS 10, which applies a single consolidation model using a definition of "control" that requires an investor (as defined in IFRS 10) to consolidate an investee (as defined in IFRS 10) where: (i) the investor has power over the investee; (ii) the investor has exposure or rights to variable returns from involvement with the investee; and (iii) the investor can use its power over the investee to affect the amount of the investor's returns.
Subsequent to the IMC Restructuring, the Company analyzed the terms of the contractual agreements with Focus (including the Commercial Agreements and the Focus Agreement) in accordance with IFRS 10 to conclude whether it should continue to consolidate the accounts of Focus in its financial statements.
- 5 -
Under IFRS 10, consolidation occurs when an investor can exercise control over an investee. Control is achieved through voting rights or other evidence of power. Where there are no direct holdings, under IFRS 10, an investor (as defined in IFRS 10) needs to consider other evidence of power and ability to unilaterally direct an investee's (as defined in IFRS 10) relevant activities. In view of the contractual agreements and the guidance in IFRS 10, notwithstanding that the Company has no direct or indirect ownership of Focus, it has sufficient rights to unilaterally direct the relevant activities (a concept known as "de facto control"), mainly due to the following:
(a) the Company receiving economic benefits from Focus (and the terms of the Commercial Agreements cannot be changed without the approval of the Company);
(b) the Company having the option to purchase the divested 74% interest in Focus held by Oren Shuster, the CEO, director and a promoter of the Company, and Rafael Gabay, a consultant, former director and a promoter of the Company;
(c) Messrs. Shuster and Gabay each being a director of Focus (while Mr. Shuster concurrently being a director, officer and substantial shareholder of the Company and Mr. Gabay concurrently being a substantial shareholder of the Company); and
(d) the Company providing management and support activities to Focus through the Services Agreement.
Accordingly, under IFRS 10, the Company has "de facto control" over Focus, and therefore consolidates the financial results of Focus in the Company's financial statements.
For additional information, please see "Risk Factors - Consolidation of Focus Financial Results under IFRS 10 and Maintenance of Common Control".
CURRENCY AND EXCHANGE RATES
References in this AIF to "CAD", "$", dollars or currency are to the lawful currency of Canada, unless otherwise indicated. In addition, this AIF includes references to (i) "NIS" which means the New Israeli Shekel, the lawful currency of the State of Israel. As of April 23, 2021, the value of one Canadian dollar expressed in NIS, based on the exchange rate available through the Bank of Israel, is NIS 2.6078, and (ii) "USD" which means the United States Dollar, the lawful currency of the United States of America. As of April 16, 2021, the value of one Canadian dollar expressed in USD, based on the exchange rate available through the U.S. Federal Reserve, is USD 0.8001; (iii) "EUR" which means the Euro, the lawful currency of the European Union. As of April 23, 2021, the value of one Canadian dollar expressed in EUR, based on the exchange rate available through the European Central Bank, is EUR 0.6639.
GLOSSARY OF TERMS
Unless otherwise indicated, the following terms used in this Annual Information Form shall have the meanings ascribed to them as set forth below:
"1961 Single Convention on Narcotic Drugs" means the Single Convention on Narcotic Drugs, 1961, an international treaty regarding the international control of narcotic drugs.
"ACMPR" means Access to Cannabis for Medical Purposes Regulations;
"Adjupharm" means Adjupharm GmbH, a company incorporated under the laws of Germany;
"Adjupharm Licenses" has the meaning set out in "Description of the Business - Production, Distribution and Sales in Principal Markets - Europe";
- 6 -
"AMG" has the meaning set out in "Medical Cannabis Regulatory Framework in Israel and Germany - Germany";
"BCBCA" means the Business Corporations Act (British Columbia), as amended, including all regulations promulgated thereunder;
"BfArM" has the meaning set out in "Medical Cannabis Regulatory Framework in Israel and Germany - Germany - Cultivation in Germany and Distribution of Medical Cannabis Cultivated in Germany";
"Board" means the board of directors of the Company as presently constituted;
"Broker Options" means broker compensation options of the Company;
"BtMG" has the meaning set out in "Medical Cannabis Regulatory Framework in Israel and Germany - Germany";
"Cannabis Act" means the Cannabis Act (Canada), as amended, including all regulations promulgated thereunder.
"Cannabis Agency" has the meaning set out in "Medical Cannabis Regulatory Framework in Israel and Germany - Germany - Cultivation in Germany and Distribution of Medical Cannabis Cultivated in Germany";
"Cannabis License" and "Cannabis Licenses", respectively, have the meanings set out in "Medical Cannabis Regulatory Framework in Israel and Germany - Israel - Licensing and Authorization for Commercial Activities in the Medical Cannabis Field";
"cannabis oil" means the extract of cannabis inflorescence diluted with oil;
"Cannabis Regulations" has the meaning set out in "Description of the Business - Recreational Cannabis Regulatory Framework in Canada";
"CBD" means cannabidiol;
"CBN" means cannabinol;
"CEO" means chief executive officer;
"CFO" means chief financial officer;
"Commercial Agreements" has the meaning set out in "Corporate Structure - Intercorporate Relationships";
"Common Shares" means at any particular time the issued and outstanding common shares in the capital of the Company at that time;
"Company" means IM Cannabis Corp., a corporation continued under the BCBCA with its registered office located in Vancouver, British Columbia;
"Consolidation" has the meaning set out in "General Development of the Business - Recent Developments - Developments Following the Financial Year Ended December 31, 2020";
- 7 -
"Construction Allegations" has the meaning set out in "Risk Factors - Reliance on Focus Facility";
"COVID-19" means the COVID-19 novel coronavirus;
"CSA Staff Notice 51-352" means Staff Notice 51-352 (Revised) - Issuers with U.S. Marijuana-related Activities of the Canadian Securities Administrators.
"CSE" means the Canadian Securities Exchange;
"Dangerous Drugs Ordinance" means the Dangerous Drugs Ordinance [New Version], 1973 [Hebrew];
"Directive 150" means Directive 150/2016 - IMC-GSP certification, the IMCA directive that sets the standards for the security and protection measures that must be taken throughout the entire supply chain of medical cannabis;1
"Directive 151" means Directive 151/2016 - IMC-GAP certification, the IMCA directive that sets the norms and standards for growing medical cannabis in Israel;2
"Directive 152" means Directive 152/2016 - IMC-GMP certification, the IMCA directive that provides the IMC-GMP rules and standards for the creation and production of medical cannabis goods in Israel;3
"Directive 153" means Directive 153/20163 - IMC-GDP certification, the IMCA directive that sets the conditions for the proper storage and delivery of medical cannabis products in Israel;4
"Dutch Tender" has the meaning set out in "General Development of the Business - Recent Developments - Developments During the Financial Year Ended December 31, 2020";
"EU" means the European Union;
"EU-GACP Standard" means the good agricultural and collection practice standard set out by the European Union and coordinated by the European Medicines Agency for companies that cultivate, harvest and collect cannabis to manufacture, process, package and store;
"EU-GMP Standard" means the good manufacturing practice standard set out by the European Union and coordinated by the European Medicines Agency for manufacturers of medical products intended for the European Union market;
"EUR" has the meaning set out in "Currency and Exchange Rates";
"Export Guidelines" has the meaning set out in "Medical Cannabis Regulatory Framework in Israel and Germany - Israel - Medical Cannabis Exports";
"Export Resolution" has the meaning set out in "Medical Cannabis Regulatory Framework in Israel and Germany - Israel - Medical Cannabis Exports";
"Final Shelf Prospectus" has the meaning set out in "General Development of the Business - Recent Developments - Developments Following the Financial Year Ended December 31, 2020";
1 Directive 150 [Hebrew] - https://www.health.gov.il/hozer/mmk150_2016.pdf
2 Directive 151 [Hebrew] - https://www.health.gov.il/hozer/mmk151_2016.pdf
3 Directive 152 [Hebrew] - https://www.health.gov.il/hozer/mmk152_2016.pdf
4 Directive 153 [Hebrew] - https://www.health.gov.il/hozer/mmk153_2016.pdf
- 8 -
"Focus" means Focus Medical Herbs Ltd., a company incorporated under the laws of the State of Israel;
"Focus Agreement" has the meaning set out in "Corporate Structure - Intercorporate Relationships";
"Focus Facility" means the propagation and cultivation facility in Moshav Sde Avraham, Israel, operated by Focus pursuant to the Focus Lease Agreement;
"Focus Lease Agreement" means the long-term land lease agreements between Focus and the landowners on which the Focus Facility is built and operated;
"Focus License" has the meaning set out in "Description of the Business - Production, Distribution and Sales in Principal Markets - Israel";
"Galen" means Galen Industries Single Member Societe Anonyme;
"GDPR" means the General Data Protection Regulation (EU) 2016/679;
"German Local Tender" has the meaning set out in "Medical Cannabis Regulatory Framework in Israel and Germany - Germany - Cultivation in Germany and Distribution of Medical Cannabis Cultivated in Germany";
"Group" means, collectively, the Company, its subsidiaries, and Focus, an Israeli private company over which IMC Holdings exercises "de facto control" under IFRS 10;
"IFRS" means International Financial Reporting Standards as issued by the International Accounting Standards Board applicable as at the relevant date;
"IFRS 10" means IFRS 10 Consolidated Financial Statements, the reporting standard under IFRS outlining the requirements for the preparation and presentation of consolidated financial statements when an entity controls one or more other entities;
"IMC-GAP" or "GAP Standard" means the good agricultural practices standard set out by the IMCA in Directive 151, and is required for Israeli cultivation and propagation licenses;
"IMC-GDP" or "GDP Standard" means the good manufacturing practices standard set out by the IMCA in Directive 153, and is required for Israeli transportation, storage and distribution licenses;
"IMC-GMP" or "GMP Standard" means the good manufacturing practices standard set out by the IMCA in Directive 152, and is required for Israeli manufacturing licenses;
"IMC-GSP" or "GSP Standard" means the good security practices standard set out by the IMCA in Directive 150, and is required throughout the Israeli supply chain for cannabis-related activities;
"IMC Holdings" means I.M.C. Holdings Ltd., a limited liability company existing under the laws of the State of Israel;
"IMC Netherlands Holdco" has the meaning set out in "General Development of the Business - Recent Developments - Developments During the Financial Year Ended December 31, 2020";
"IMC Restructuring" has the meaning set out in "Corporate Structure - Intercorporate Relationships";
"IMCA" means the Israeli Medical Cannabis Agency, an agency operated by the MOH;
- 9 -
"IP Agreement" has the meaning set out in "Corporate Structure - Intercorporate Relationships";
"IT systems" has the meaning set out in "Risk Factors - Information Technology";
"kg" means a kilogram;
"Listed Warrants" has the meaning set out in "Description of Capital Structure - Warrants";
"MediPharm Labs" has the meaning set out in "General Development of the Business - Recent Developments - Developments Following the Financial Year Ended December 31, 2020";
"MGC" has the meaning set out in "General Development of the Business - Recent Developments - Developments During the Financial Year Ended December 31, 2020";
"MOH" means the Israeli Ministry of Health;
"MOH Allegations" has the meaning set out in "Risk Factors - Reliance on Focus Facility";
"MOH Regulations" means the Dangerous Drugs Ordinance, any amendments of the Dangerous Drugs Ordinance, any regulations enacted by virtue of the Dangerous Drugs Ordinance from time to time, and the regulatory regime introduced by the MOH with respect to the medical cannabis industry in Israel, including the Road Map, Procedure 106, Procedure 109, the Export Resolution and the Export Guidelines;
"NASDAQ" means the NASDAQ Capital Market;
"NGC" has the meaning set out in "General Development of the Business - Recent Developments - Developments Following the Financial Year Ended December 31, 2020";
"NGC Supply Agreement" has the meaning set out in "General Development of the Business - Recent Developments - Developments Following the Financial Year Ended December 31, 2020";
"NI 52-110" means National Instrument 52-110 - Audit Committees;
"NIS" has the meaning set out in "Currency and Exchange Rates";
"NMCP" has the meaning set out in "Medical Cannabis Regulatory Framework in Israel and Germany - Israel";
"OBCA" means the Business Corporations Act (Ontario), as amended, including all regulations promulgated thereunder;
"Option Cap" has the meaning set out in "Description of Capital Structure - Options";
"Options" means incentive stock options to purchase Common Shares granted to certain eligible participants of the Company in accordance with the terms of the Stock Option Plan;
"Person" means an individual, partnership, unincorporated association, unincorporated syndicate, unincorporated organization, trust, trustee, executor, administrator or other legal representative;
"Pilot Program" has the meaning set out in "Medical Cannabis Regulatory Framework in Israel and Germany - Israel - Medical Cannabis Exports";
- 10 -
"PIPEDA" means the Personal Information Protection and Electronic Documents Act (Canada);
"Preliminary Shelf Prospectus" has the meaning set out in "General Development of the Business - Recent Developments - Developments Following the Financial Year Ended December 31, 2020";
"Procedure 106" has the meaning set out in "Medical Cannabis Regulatory Framework in Israel and Germany - Israel - Patient Medical Use";
"Procedure 109" has the meaning set out in "Medical Cannabis Regulatory Framework in Israel and Germany - Israel - Medical Cannabis Imports";
"Qualified Securities" has the meaning set out in "General Development of the Business - Recent Developments - Developments Following the Financial Year Ended December 31, 2020";
"Registration Statement" has the meaning set out in "General Development of the Business - Recent Developments - Developments Following the Financial Year Ended December 31, 2020";
"Reverse Takeover Transaction" has the meaning set out in "General Development of the Business - Recent Developments - Developments Following the Reverse Takeover Transaction";
"Road Map" has the meaning set out in "Medical Cannabis Regulatory Framework in Israel and Germany - Israel - Licensing and Authorization for Commercial Activities in the Medical Cannabis Field";
"RSU" has the meaning set out in "Description of Capital Structure - Restricted Share Units";
"RSU Plan" has the meaning set out in "Description of Capital Structure - Restricted Share Units";
"SEC" means the United States Securities and Exchange Commission;
"Securities Commissions" has the meaning set out in "General Development of the Business - Recent Developments - Developments Following the Financial Year Ended December 31, 2020";
"Services Agreement" has the meaning set out in "Corporate Structure - Intercorporate Relationships";
"Shiran" means Shiran Single Member Societe Anonyme;
"Stock Option Plan" has the meaning set out in "Description of Capital Structure - Options";
"Subscription Receipts" has the meaning set out in "General Development of the Business - Recent Developments - The Reverse Takeover Transaction";
"THC" means tetrahydrocannabinol;
"TJAC" has the meaning set out in "General Development of the Business - Recent Developments - Developments Following the Financial Year Ended December 31, 2020";
"TJAC Facilities" has the meaning set out in "Production, Distribution and Sales in Principal Markets - Canada";
"TJAC Leases" has the meaning set out in "Production, Distribution and Sales in Principal Markets - Canada";
"Trichome" has the meaning set out in "General Development of the Business - Recent Developments - Developments Following the Financial Year Ended December 31, 2020";
"Trichome Transaction" has the meaning set out in "General Development of the Business - Recent Developments - Developments Following the Financial Year Ended December 31, 2020";
- 11 -
"Unlisted Warrants" has the meaning set out in "Description of Capital Structure - Warrants";
"U.S." means the United States of America;
"USD" has the meaning set out in "Currency and Exchange Rates";
"Warrants" means Common Share purchase warrants of the Company and includes Listed Warrants and Unlisted Warrants; and
"Xinteza" has the meaning set out in "General Development of the Business - Developments Following the Reverse Takeover Transaction";
Words importing the singular number only include the plural and vice versa, and words importing any gender include all genders.
- 12 -
CORPORATE STRUCTURE
Name, Address and Incorporation
The full corporate name of the Company is "IM Cannabis Corp." The Company's head office is located at Kibbutz Glil Yam, Israel and its registered office is located at 550 Burrard Street, Suite 2300, Bentall 5, Vancouver, British Columbia, V6C 2B5, Canada. The Company is a reporting issuer under the laws of each of the Provinces and Territories of Canada.
The Company was incorporated as "Nirvana Oil & Gas Ltd." pursuant to a Certificate of Incorporation issued under the BCBCA on March 7, 1980. Effective July 12, 2013, in connection with a share consolidation, the Company changed its name to "Navasota Resources Inc."
On June 22, 2018, the Company completed a consolidation of its Common Shares on the basis of one (1) post-consolidation Common Share for every 5 pre-consolidation Common Shares.
On October 4, 2019, in connection with the Reverse Takeover Transaction, the Company effected a consolidation of its Common Shares on the basis of one (1) post-consolidation Common Share for every 2.83 pre-consolidation Common Shares and changed its name to "IM Cannabis Corp."
On February 12, 2021, in connection with its NASDAQ listing application, the Company effected the Consolidation on the basis of one (1) post-Consolidation Common Share for every four (4) pre-Consolidation Common Shares.
Intercorporate Relationships
The organizational chart of the Company, including the governing law or the jurisdiction of organization of the Company and each material subsidiary and the percentage of voting securities beneficially owned, or controlled or directed, directly or indirectly, by the Company, is set out below.
- 13 -
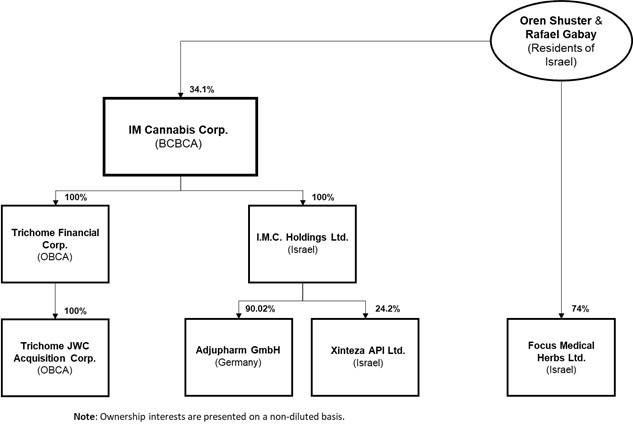
Current Israeli law requires the prior approval by the IMCA of the identity of any shareholder owning 5% or more of an Israeli company licensed to engage in cannabis-related activities. For a number of reasons, including the opportunity to leverage a network of multiple Israeli licensed producers cultivating under the IMC brand, and in contemplation of a "go-public transaction" to geographically diversify the Company's share ownership, IMC Holdings restructured its organization on April 2, 2019 (the "IMC Restructuring") resulting in the divestiture to Oren Shuster and Rafael Gabay of its interest in Focus, which is licensed by the MOH to propagate and cultivate cannabis in Israel:
IMC Holdings retains an option with Messrs. Shuster and Gabay to re-acquire the sold interest in Focus at its sole discretion and in accordance with Israeli cannabis regulations, within 10 years of the date of the IMC Restructuring (the "Focus Agreement"). The Focus Agreement sets an aggregate exercise price equal to NIS 765.67 per share of Focus for a total consideration of NIS 2,756,500, that being equal to the price paid by Messrs. Shuster and Gabay for the acquired interests in Focus at the time of the IMC Restructuring. Although the Company does not hold any voting interests in Focus, the Company is permitted to consolidate the accounts of Focus in its financial statements by virtue of its "de facto" control over Focus in accordance with IFRS 10. For more information on the Company's corporate structure with respect to Focus and the Company's accounting practices, please see "Note Regarding the Company's Accounting Practices".
As part of the IMC Restructuring, IMC Holdings and Focus entered into: (i) an agreement in which Focus would use the IMC brand on an exclusive basis for the sale of any cannabis plant and/or cannabis product produced by Focus, either alone or together with other sub-contractors engaged by Focus, among other rights and services (the "IP Agreement"); and (ii) an agreement pursuant to which Focus would use IMC Holdings for certain management and consulting services including: (a) business development services; (b) marketing services; (c) strategic advisory services; (d) locating potential collaborations on a worldwide basis; and (e) financial analysis services (the "Services Agreement" and collectively with the IP Agreement, the "Commercial Agreements").
- 14 -
GENERAL DEVELOPMENT OF THE BUSINESS
The following discussion covers key events during the Company's historical development over the last three completed financial years, as well as certain subsequent events to the date of this Annual Information Form.
History prior to the Reverse Takeover Transaction
The Company historically engaged in mineral resource exploration activities but ceased operations in March 2018 to focus on identifying and evaluating new business opportunities.
The Reverse Takeover Transaction
On October 11, 2019, the Company completed a business combination with IMC Holdings resulting in a reverse takeover of the Company by shareholders of IMC Holdings (the "Reverse Takeover Transaction"). The Reverse Takeover Transaction was effected by way of a "triangular merger" between the Company, IMC Holdings and a wholly-owned subsidiary of the Company pursuant to Israeli statutory law. The Board and management of the Company were reconstituted and subsequently led by Oren Shuster. As a result of the Reverse Takeover Transaction, the Company changed its business from mining to the international medical cannabis industry.
In connection with the Reverse Takeover Transaction, the Company completed a private placement offering of 19,460,527 subscription receipts ("Subscription Receipts") of a wholly-owned subsidiary of the Company at a price of $1.05 per Subscription Receipt for aggregate gross proceeds of approximately $20.4 million. Upon completion of the Reverse Takeover Transaction, each Subscription Receipt was exchanged for one unit comprised of one (1) Common Share and one-half of one (1/2) Warrant. Each whole Warrant was exercisable for one Common Share at an exercise price of $1.30 for a period of 24 months following the closing of the Reverse Takeover Transaction.
On November 5, 2019, the Common Shares began trading on the CSE under the ticker symbol "IMCC".
On November 19, 2019, the Warrants began trading on the CSE under the ticker symbol "IMCC.WT".
Developments Following the Reverse Takeover Transaction
On December 26, 2019, IMC Holdings entered into a share purchase agreement with Xinteza API Ltd. ("Xinteza"), a company with a unique biosynthesis technology, whereby the Company acquired, on an as-converted and fully diluted basis, 25.37% of Xinteza's outstanding share capital, for consideration of US$1,700,000 (approximately $2,223,000, according to the December 24, 2019 exchange rate published by the Bank of Canada) paid in several installments (the "Xinteza SPA"). As of September 30, 2020, the Company has paid all outstanding installments pertaining to the Xinteza SPA. As of December 31, 2020, following further investments by third parties into Xinteza, the Company holds 24.2% of the outstanding share capital of Xinteza on an as-converted and fully diluted basis. Under an exclusive license from Yeda Research & Development Company Ltd., the commercial division of the Weizmann Institute of Science, and based on disruptive plant genetics and metabolomics research led by Professor Asaph Aharoni, Xinteza has been developing advanced proprietary technologies related to the production of cannabinoid-based active pharmaceutical ingredients for the pharmaceutical and food industries using biosynthesis and bio-extraction technologies.
- 15 -
Recent Developments
Developments During the Financial Year Ended December 31, 2020
On January 23, 2020, IMC Holdings entered into definitive agreements to establish a medical cannabis cultivation and processing joint venture in Greece with Galen, a Greek company established by a consortium of investors in Greece with extensive experience in the pharmaceutical, media, finance and energy sectors. As a result of the agreements, IMC Holdings acquired ownership of 25% of the paid-up capital of Shiran, a private company incorporated and registered in Greece and originally wholly-owned by Galen, while the remaining 75% remained under the ownership of Galen. Under the agreements, each party is committed to fund the initial capital expenditures, totaling approximately up to EUR 8,000,000 for the construction of an EU-GMP certified cultivation and processing facility in Greece.
Also on January 23, 2020, Shiran, Galen and IMC Holdings signed a preferred supply agreement (the "Galen Supply Agreement"). Under the Galen Supply Agreement, IMC Holdings has the right to purchase up to 25% of the total production of Shiran at a preferred price as determined therein, for an initial period of five years. As of the date of this Annual Information Form, no material capital expenditures have been made towards Shiran given the uncertainty relating to COVID-19 and the Company is deferring any further investment into Greece indefinitely.
On March 17, 2020, the Company held its annual general shareholders' meeting. At the meeting, incumbent director Jesse Kaplan did not seek re-election as a director of the Company and Vivian Bercovici and Rafael Gabay were elected to the Board.
On March 23, 2020, Focus signed a supply agreement (the "Intelicanna Supply Agreement") with Intelicanna Ltd. ("Intelicanna") for the purchase by Focus of a minimum of 500kg and up to 1,000kg of medical cannabis cultivated by Intelicanna. Additional purchases may be made by Focus under the Intelicanna Supply Agreement without a change to the contracted price paid to Intelicanna. The finished products are to be sold to pharmacies in Israel under the IMC brand. The Intelicanna Supply Agreement is in effect for a term of 12 months from the date of the first planting in Intelicanna's facility. Intelicanna has received access to some of Focus' unique and proprietary genetics for the sole purpose of delivering product under the Intelicanna Supply Agreement; however, the genetics remain the exclusive property of Focus. Under the Intelicanna Supply Agreement, Intelicanna is responsible for all production activities under Focus' supervision and quality control practices throughout the growing process at Intelicanna's site.
On March 30, 2020, Focus signed a binding three-year sales agreement for the sale of IMC-branded medical cannabis products (the "March 2020 Pharmacy Sales Agreement") to three pharmacies in Jerusalem operating under the Oranim Pharm and Medi Plus banners. Pursuant to the March 2020 Pharmacy Sales Agreement, Focus is to supply such pharmacies with a total of 800kg of medical cannabis products annually for a period of three years, commencing in 2021, for an aggregate amount of 2,400kg of medical cannabis products at a contracted price.
On March 31, 2020, Focus signed a supply agreement with Way of Life Ltd., an IMC-GAP certified cultivator ("Way of Life"), and Cannation Ltd., an IMC-GAP applicant ("Cannation", and together with Way of Life, the "Suppliers") to purchase a total of approximately 2,600kg of medical cannabis per year for an aggregate amount of up to 7,800kg of medical cannabis products over three years. Of the aggregate amount to be supplied under the agreement, delivery of 6,200kg was contingent upon Cannation receiving its IMC-GAP certification. All finished products produced from the medical cannabis supplied under such supply agreement will be sold under the IMC brand to pharmacies in Israel. Under the supply agreement, the Suppliers obtained access to some of Focus' unique and proprietary genetics for the sole purpose of cultivating and delivering medical cannabis under the supply agreement; however, the genetics would remain the exclusive property of Focus. In addition, Focus received access to the Suppliers' growing facilities to monitor the entire growing process. As Focus has secured the necessary supply to fulfill its delivery obligations under its pharmacy sales agreements and support its Israeli operations, and following the expiration of the milestone for Cannation to obtain IMC-GAP certification, the supply agreement with Cannation was terminated on November 24, 2020.
- 16 -
On April 2, 2020, the Company announced that Adjupharm had received the necessary approvals from regulatory authorities to begin imports and sales of medical cannabis products under the IMC brand to German patients.
On April 6, 2020, Focus signed a binding two-year sales agreement for the sale of medical cannabis products under the IMC brand with Shor Tabachnik pharmacies ("Tabachnik") (the "Tabachnik Sales Agreement"). According to the Tabachnik Sales Agreement, Focus will sell to Tabachnik 1,000kg of medical cannabis products under the IMC brand annually for the duration of the Tabachnik Sales Agreement at an agreed upon price beginning in 2021.
On April 13, 2020, Focus signed a binding three-year agreement for the sale of 13,575kg of medical cannabis products under the IMC brand to Super-Pharm (Israel) Ltd., the largest pharmacy chain in Israel ("Super-Pharm") (the "SP Sales Agreement"). According to the SP Sales Agreement, Focus will sell to Super-Pharm a total of 13,575kg of medical cannabis products under the IMC brand over the next three years. Medical cannabis products sold under the SP Sales Agreement will include both dry inflorescence and extract products at an agreed upon price.
On April 13, 2020, Focus signed a one-year binding agreement for the sale of 1,000kg of medical cannabis products under the IMC brand to Panaxia Labs Israel, Ltd. at an agreed upon price.
On April 14, 2020, Focus signed an agreement for the sale of up to 1,500kg of medical cannabis products under the IMC brand to Max Pharm Ltd. ("Max Pharm") over a three year period (the "MP Sales Agreement"). Under the MP Sales Agreement, Focus will sell to Max Pharm a total of 500kg of medical cannabis products under the IMC brand annually at an agreed upon price beginning in 2021. Max Pharm has an option to purchase an additional 500kg of medical cannabis products from Focus in each of 2021, 2022 and 2023, for a total volume of up to 3,000kg over three years.
On April 21, 2020, Focus signed a binding three-year agreement for the sale of 12,600kg of medical cannabis products under the IMC brand to PharmYarok Ltd. ("PharmYarok") (the "PY Sales Agreement"). According to the PY Sales Agreement, Focus will sell to PharmYarok a total of 12,600kg of medical cannabis products under the IMC brand between 2021 and 2023 at an agreed upon price, subject to PharmYarok meeting certain regulatory requirements. Medical cannabis products sold under the PY Sales Agreement may include both dry inflorescence and extract products.
On April 26, 2020, Focus signed a three-year definitive supply agreement (the "Megadim Supply Agreement") with an IMC-GAP certified independent farmer located in Megadim, Israel and licensed to cultivate medical cannabis. Under the Megadim Supply Agreement, Focus will purchase a total of up to 8,060kg of medical cannabis over three years at an agreed upon price, of which approximately 7,500kg was contingent upon the supplier meeting quality criteria set under the Megadim Supply Agreement. All finished products created from the medical cannabis pursuant to the Megadim Supply Agreement will be sold by Focus under the IMC brand to pharmacies in Israel. On February 10, 2021, the Company announced the amendment to the Megadim Supply Agreement, to reflect the supply of only three harvests of medical cannabis being purchased by Focus. Under such amendment and subject to the terms therein, upon payment for all three harvests, the Megadim Supply Agreement will be terminated. Following this change, approximately 570 kg of medical cannabis are to be provided to Focus.
- 17 -
On May 7, 2020, the Company announced that Adjupharm received purchase orders for an aggregate of 360kg of IMC-branded medical cannabis products pursuant to certain distribution agreements entered into with German distributors in March 2020.
On May 8, 2020, Adjupharm received regulatory confirmation for the import of up to 5,800kg of medical cannabis products into Germany from foreign suppliers under the Adjupharm Licenses within a 12-month period. Such confirmation allows Adjupharm to import either bulk products, such as dry inflorescences and dronabinol, or extract products for end-products, at specified quantities set out in the confirmation.
On May 12, 2020, the Company announced that Adjupharm received a purchase commitment from a distributor in Germany for 465kg of IMC-branded medical cannabis products over a 12-month period.
On May 26, 2020, Focus received its first shipment of 200kg of imported medical cannabis from Spain-based Linneo Health S.L, the Company's EU-GMP certified supply partner for medical cannabis, to be sold in Israel under the IMC brand starting in June 2020.
On June 12, 2020, the Company signed a binding term sheet for the exclusive distribution rights of CannEpil® in Israel for a period of five years (the "CannEpil Term Sheet"), subject to CannEpil® meeting requirements under applicable laws to be qualified as a legal drug in Israel. CannEpil® is a phytocannabinoid medicine developed by MGC Pharmaceuticals Ltd. ("MGC") for the treatment of refractory epilepsy. According to the CannEpil Term Sheet, IMCC would be responsible for the registration, promotion and distribution of CannEpil® in Israel. IMCC would also obtain all necessary permits and licenses for importation and commercialization. MGC would continue to own all intellectual property rights associated with CannEpil® and its continued research and development.
On June 18, 2020, Focus received its first imported shipment of medical cannabis from a Canadian EU-GMP certified medical cannabis cultivator. The shipment was comprised of approximately 200kg of medical cannabis to be sold by Focus under the IMC brand to pharmacies in Israel.
In July 2020, Adjupharm entered into several binding medical cannabis sales agreements with the following distributors in Germany: Zur Rose Pharma GmbH ("Zur Rose"), Axicorp Group, Canymed GmbH and Materia Deutschland GmbH. These additional distributors brought Adjupharm's total number of contracted German distributors to seven, with definitive purchase commitments with such distributors totaling 1,525kg of medical cannabis products bearing the IMC brand to be delivered in Germany over a 12-month period. A settlement to terminate the medical cannabis sales agreement with Zur Rose was reached on March 30, 2021.
On July 24, 2020, Focus signed a supply agreement with Ever Green Solomon Pharma Ltd ("Ever Green") (the "Ever Green Supply Agreement"), an IMC-GAP certified cultivator, for the purchase of all of the medical cannabis production cultivated by Ever Green in an 86,000 square feet area of its facility, over a period of five years, with an option for Focus to extend the term by an additional five years, for a total term of up to 10 years. The finished products created from medical cannabis delivered pursuant to the Ever Green Supply Agreement will be sold by Focus to pharmacies in Israel under the IMC brand.
On July 28, 2020, the Company established a wholly-owned subsidiary in the Netherlands, IM Cannabis Holding NL B.V. ("IMC Netherlands Holdco"), which subsequently established another Dutch entity, IMC Holland B.V. ("Holland B.V."), in which 60% is owned by IMC Netherlands Holdco, and the remaining 40% is owned by a group of four individuals with expertise in the Dutch cannabis market. Holland B.V. was incorporated for the purpose of applying for a Dutch governmental tender (the "Dutch Tender") and to establish a full cannabis supply chain to coffee shops in the Dutch municipalities participating in the Dutch Tender. On November 27, 2020, the Company received notice that its application for the Dutch Tender was not accepted. Accordingly, Holland B.V. was liquidated effective as of December 18, 2020. As of the date of this Annual Information Form, the Company is exploring other strategic opportunities involving successful applicants of the Dutch Tender but does not currently have any material operations in the jurisdiction.
- 18 -
On September 8, 2020, Adjupharm signed distribution agreements for the sale of IMC-branded medical cannabis products with Cansativa GmbH and Ilios Sante GmbH.
On September 9, 2020, Adjupharm signed a distribution agreement for the sale of IMC-branded medical cannabis products with Farmako GmbH, bringing its total number of contracted German distributors to ten.
On September 15, 2020, the Company imported its first shipment of medical cannabis from its EU-GMP supply partner into Germany for distribution and sale through its German distributors, under the IMC brand.
On September 23, 2020, the Company officially launched the IMC brand in Germany as four of the Company's German distribution partners received shipments of medical cannabis products for sale in the German medical cannabis market. The first product bearing the IMC brand available to customers was the High THC T20/1 medical cannabis inflorescences.
On October 8, 2020, the Company applied to list its Common Shares on the NASDAQ Capital Market under the trading symbol "IMCC", subject to the satisfaction of all applicable listing and regulatory requirements, including registration of the Common Shares with the SEC, and satisfaction of NASDAQ listing requirements.
On December 16, 2020, the Company's shareholders approved a special resolution authorizing a share consolidation of the Common Shares at a ratio of between three (3) and eight (8) pre-consolidation Common Shares for every one post-consolidation Common Share, to be implemented at the discretion of the Board.
On December 29, 2020, Marc Lustig was appointed as Executive Chairman of the Company.
On December 30, 2020, the Company entered into a definitive agreement with Trichome Financial Corp. ("Trichome"), to combine their businesses pursuant to a plan of arrangement to be completed under the OBCA (the "Trichome Transaction").
Developments Following the Financial Year Ended December 31, 2020
On January 26, 2021, the Company announced that it received confirmation from The Depository Trust Company ("DTC") that its Common Shares are eligible for electronic clearing and settlement through DTC in the U.S.
On February 12, 2021, the Company consolidated all of its issued and outstanding Common Shares on a four (4) to one (1) basis (the "Consolidation"). Following the Consolidation, the number of Listed Warrants outstanding was not altered; however, the exercise terms were adjusted such that four Listed Warrants are exercisable for one Common Share following the payment of an adjusted exercise price of $5.20.
- 19 -
On February 22, 2021, the Company appointed Brian Schinderle and Haleli Barath to the Board. Both Mr. Schinderle and Ms. Barath are independent directors under applicable Canadian and United States securities laws. Concurrently with these appointments, Rafael Gabay and Steven Mintz resigned from the Board.
On March 1, 2021, the Company's Common Shares commenced trading on NASDAQ under the ticker symbol "IMCC", making the Company the first Israeli medical cannabis operator to list its shares on NASDAQ.
On March 8, 2021, the Company announced that Focus signed a multi-year supply agreement with GTEC Holdings Ltd. ("GTEC"), a Canadian licensed producer of handcrafted and high quality cannabis (the "GTEC Agreement"). According to the GTEC Agreement, Focus will import GTEC's high-THC medical cannabis inflorescence into Israel to be sold under the IMC brand. With the arrival of these commercial shipments, the Company will launch a new category of imported premium indoor medical cannabis products under its well-established brand. The import of the Canadian-grown high-THC strains from GTEC's subsidiary, Grey Bruce Farms Incorporated ("GBF"), is expected to commence in Q2 2021, subject to fulfilling all regulatory requirements in relation to such import, including compliance with MOH regulations and receipt of a valid export license from Health Canada. According to the GTEC Agreement, Focus will purchase a minimum quantity of 500kg of high-THC medical cannabis inflorescence from GBF and will be the exclusive recipient of GTEC cannabis products in the Israeli market for a period of 12 months from the date that the first shipment of GTEC products arrives in Israel (the "Exclusive Term"). The Exclusive Term can be extended under the terms of the GTEC Agreement by an additional 6 months.
On March 12, 2021, the Company filed a preliminary short form base shelf prospectus (the "Preliminary Shelf Prospectus") with the securities commissions or similar securities regulatory authorities in each of the provinces and territories of Canada (the "Securities Commissions"), and on March 15, 2021, the Company filed a corresponding shelf registration statement on Form F-10, with the SEC under the Multijurisdictional Disclosure System ("MJDS") established between Canada and the United States.
On March 12, 2021, Adjupharm entered into a supply agreement with Northern Green Canada Inc. ("NGC") (the "NGC Supply Agreement"). Under the terms of the NGC Supply Agreement, NGC will provide Adjupharm with three new strains of medical cannabis products, to be distributed under the IMC brand to German pharmacies pursuant to Adjupharm's distribution agreements with its German distribution partners. Shipments from NGC are expected to commence in Q2 2021.
On March 18, 2021, the Company acquired all of Trichome's issued and outstanding shares (the "Trichome Shares") and closed the Trichome Transaction that was previously announced on December 30, 2020. Pursuant to the terms of the Trichome Transaction, former holders of Trichome Shares and former holders of Trichome convertible instruments (the "Trichome Securityholders") received 0.24525 of a Common Share for each Trichome Share held and each in-the-money convertible instrument of Trichome. As a result of the Trichome Transaction, a total of 10,104,901 Common Shares were issued to the Trichome Securityholders, resulting in former Trichome Securityholders holding approximately 20.06% of the total number of issued and outstanding Common Shares immediately after closing. In addition, 100,916 Common Shares were issued to financial advisors for advisory fees in connection with the Trichome Transaction.
- 20 -
On March 29, 2021, Adjupharm entered into a supply agreement with MediPharm Labs Corp. ("MediPharm Labs") for certain medical cannabis extract products to be delivered by MediPharm Labs over an initial two-year term with an automatic two-year extension period.
On March 31, 2021, in connection with the Preliminary Shelf Prospectus, the Company filed a final short form base shelf prospectus (the "Final Shelf Prospectus") with the Securities Commissions and a corresponding shelf registration statement on Form F-10 (the "Registration Statement") with the SEC. The Final Shelf Prospectus and the Registration Statement enable the Company to offer up to USD 250,000,000 (or its equivalent in other currencies) of Common Shares, warrants, subscription receipts, debt securities, units (collectively, the "Qualified Securities"), or any combination of such Qualified Securities from time to time, during the 25-month period that the Final Shelf Prospectus is effective. The specific terms of any offering under the Final Shelf Prospectus and the intended use of the net proceeds will be established in a prospectus supplement, which will be filed with the Securities Commissions and the SEC in connection with any such offering.
In March 2021, Adjupharm entered into two supply agreements with supply partners in China, under which Adjupharm shall buy COVID-19 rapid antigen test kits. Concurrently, Adjupharm entered into several resale agreements with reseller partners in Germany, under which Adjupharm shall sell the COVID-19 antigen test kits supplied from the China-based suppliers, to be distributed to pharmacies and retailers in Germany.
On April 1, 2021, the Company entered into a definitive agreement to acquire MYM Nutraceuticals Inc. ("MYM"), pursuant to a plan of arrangement to be completed under the OBCA (the "MYM Transaction"). MYM is a Canadian cultivator, processor, and distributor of premium cannabis via its two wholly owned subsidiaries - Highland Grow Inc., in Antigonish, Nova Scotia and SublimeCulture Inc., in Laval, Quebec. Under the terms of the MYM Transaction, the shareholders of MYM will receive 0.022 Common Shares for each common share of MYM. Upon completion of the MYM Transaction, former MYM shareholders will own approximately 14.5% of the Company. The completion of the MYM Transaction is expected to occur before the end of 2021, and it will be subject to required court, securityholder and regulatory approvals.
DESCRIPTION OF THE BUSINESS
General
The Company is a multi-country operator in the medical and recreational cannabis sector headquartered in Israel and with operations in Israel, Germany and Canada.
In Israel, IMC Holdings built the IMC brand of premium medical cannabis products which have been cultivated over the last decade by Focus, an Israeli licensed cultivator over which IMC Holdings exercises "de facto control" under IFRS 10, and its cultivation partners, and sold by Focus in the Israeli market. As part of its core Israeli business, the Company offers intellectual property-related services to the medical cannabis industry based on proprietary processes and technologies it developed for the production of medical cannabis products. The Company offers its intellectual property and consulting services to Focus pursuant to the Commercial Agreements and receives as consideration for such services a share of Focus' revenues resulting from the sales of medical cannabis products under the IMC brand. During the twelve month period ended December 31, 2020, the revenues generated by Focus formed a significant portion of the Company's total revenues. The Company is currently focused on implementing its global expansion strategy with the penetration of both the European and Canadian cannabis markets.
In Europe, the Company operates in Germany through Adjupharm, a German-based subsidiary and EU-GMP certified medical cannabis producer and distributor, which provides the Company with a platform to establish and entrench its brand in Germany and other European jurisdictions, applying the experience it gained in the Israeli market. The Company's European presence is augmented by strategic alliances with a network of certified distributors and suppliers across the continent and internationally. The Company's objective within Europe is to capitalize on the increasing demand for medical cannabis products and to bring the well-established IMC brand and its product portfolio to European patients. The Company's operating track record, accumulation of data and brand reputation in Israel is a competitive advantage to gain traction in the German and European markets and build support among physicians that prescribe medical cannabis products. The Company has engaged in exploratory operations to expand to Portugal and Greece, however it has deferred any further investment in these jurisdictions indefinitely in light of the uncertainty related to COVID-19. The Company has also engaged in exploratory efforts in the Netherlands and is seeking collaborative opportunities with successful applicants of the Dutch Tender, however it does not currently have any material operations in the jurisdiction.
- 21 -
The Company has long-term plans to expand its European operations by engaging in strategic acquisitions across Europe.
Following the successful completion of the Trichome Transaction on March 18, 2021, the Group's global platform now includes the adult-use recreational cannabis market in Canada, in addition to its established distribution channels for medical cannabis in Israel through Focus and in Germany through Adjupharm.
In Canada, the Company operates through Trichome, a Canadian-based subsidiary, and through Trichome JWC Acquisition Corp. ("TJAC") d/b/a JWC, a wholly-owned subsidiary of Trichome and Canadian federally licensed producer of cannabis products in the adult-use recreational cannabis market in Canada. TJAC acquired substantially all of the assets of James E. Wagner Cultivation Corp. on August 28, 2020, under a court supervised process pursuant to the Companies' Creditors Arrangement Act (Canada). Trichome is now focused on acquiring related assets to compliment TJAC and leveraging the knowledge, expertise and insights of its employees, management and founders. Furthermore, the Company expects TJAC's premium indoor cultivation facility in Canada to serve as a long-term source of premium cannabis supply for the Group.
The Company is focused on further implementing an aggressive and accretive acquisition strategy focusing on attractively valued and highly synergistic targets in Canada, including its proposed acquisition of MYM. Additionally, the Group is focused on diversifying its product portfolio with premium and super premium medical cannabis products, leveraging its Canadian acquisition strategy that is expected to result in additional opportunities to export premium cannabis products to both Israel and Germany.
Due to the impact of the COVID-19 pandemic on Germany in the first quarter of 2021, the Company, through Adjupharm, leveraged its established distribution platform to enter into several reseller agreements of COVID-19 antigen test kits. Such engagement of Adjupharm is expected to facilitate and further enhance its business relationship with pharmacies in Germany and support its distribution platform for medical cannabis. In light of the uncertainty related to COVID-19, the Company will examine the continued demand of the German market for such test kits prior to any further engagement relating thereto. For more information, please see "General Development of Business - Recent Developments".
The consolidated revenue of the Group has been generated from the sale of medical cannabis products to customers in Israel and Germany by Focus and Adjupharm, respectively. Following the completion of the Trichome Transaction, Trichome's revenues generated from its cannabis financing activities and subsidiary activities will also be included in such consolidated revenue.The Group does not engage in any U.S. cannabis-related activities as defined in CSA Staff Notice 51-352.
Neither the Company nor any of its subsidiaries currently hold, directly or indirectly, any licenses to engage in the propagation, cultivation, production, processing, distribution or sale of medical cannabis products in Israel. However, under IFRS 10, the Company is required to consolidate the results of Focus, an Israeli licensed propagator and cultivator of medical cannabis products. Focus operates under the regulations of medical cannabis products by the MOH through the IMCA to propagate and cultivate medical cannabis products in Israel. All of Focus' operations are performed pursuant to the Dangerous Drugs Ordinance and the related regulations issued by IMCA. While IMCC does not hold any of the Israeli licenses mentioned above and does not own Focus, it derives a significant portion of its consolidated revenues from Focus' revenue, which is primarily earned from the medical cannabis sales agreements that Focus has with various pharmacies in Israel. Furthermore, the Company has an option under the Focus Agreement to re-acquire 74% ownership of Focus. For more information, please see "Corporate Structure - Intercorporate Relationships" and "Note Regarding the Company's Accounting Practices".
- 22 -
Principal Products and Brands
"IMC" is a well-known medical cannabis brand in Israel. Leveraging its long-term success in the Israeli market, the Company launched the brand in Germany in 2020. The Company believes that the IMC brand has become synonymous with quality and consistency in the Israeli medical cannabis market and it was chosen as one of the top four favourite brands in Israel.5
In association with Focus, the Company maintains a brand portfolio that includes popular medical cannabis inflorescences such as Roma, Dairy Queen, London, Tel Aviv and Pandora Box, as well as full-spectrum cannabis extracts:

'Roma' is marketed as an elegant strain that is known for its strong impact and influence. Roma was chosen as one of the most favoured strains in Israel.6 'Tel-Aviv' is marketed as sativa dominated strain that is known for uplifting the spirit and enhancing creativity. Both Roma and Tel-Aviv contain THC, CBD, and CBN within the following ranges: 16-24% (THC), 0-7% (CBD), and CBN lower than 1.5%.
'London' is marketed as a distinct indica, which stands out due to its flavor and strong influence. 'Dairy Queen' is marketed as a rich, velvety strain with a cherry aroma that may assist with reducing stress and producing calmness. 'Pandora Box' is marketed as a sativa dominate strain, which confers a sense of spirit uplifting, energy and vitality. London, Dairy Queen, and Pandora Box contain THC, CBD, and CBN within the following ranges: 11-19% (THC), 0-5.5% (CBD), and CBN lower than 1.5%.
5 According to a survey carried out by Cannabis Magazine among 519 patients licensed by the MOH to consume medical cannabis (August 2020, Israel).
6 According to a survey carried out by Cannabis Magazine among 519 patients licensed by the MOH to consume medical cannabis (Aug2020, Israel).
- 23 -
All of the products are tested in certified labs according to MOH standards and certified before being packaged and labelled with detailed information about the THC, CBD and CBN content of each product.7
Israeli patients using IMC branded products reported8 improvements of 60% in oncology patients health status, 80.5% in rheumatoid arthritis, 58% in back pain, 42% in Post Traumatic Stress Disorder (PTSD), 38% insomnia patients sleep quality, and of other influences such as calm, appetite and libido.
In Germany, the Company sells an IMC-branded medical cannabis inflorescence products. The medical cannabis product sold in the German market is branded generically as "IMC" so as to rely on the Company's name recognition in establishing a foothold with German consumers.
In March 2021, Adjupharm launched a new medical cannabis inflorescence product with THC and CBD levels of 17% and 1%, respectively, under the IMC brand. The new product will be distributed pursuant to Adjupharm's distribution agreements with its German distribution partners.
In Canada, commencing March 18, 2021, following completion of the Trichome Transaction, the Company's product portfolio consists of primarily dried inflorescence, pre-rolled cannabis, pressed hash and kief offerings sold by TJAC under the JWC brand into the Canadian adult use recreational cannabis market. Dried inflorescence is sold primarily in 3.5 gram, 14 gram and 28 gram formats, all pre-rolls were sold in a 3 x 0.5 gram format and both hash and kief sold in 1 gram and 2 gram formats.
In 2021, TJAC will continue to offer its existing product portfolio and plans to introduce additional offerings in the form of new dried inflorescence strains, new packaging formats and a rebranding of its dried inflorescence, pre-rolled cannabis, hash and kief products under the company's recently launched Wagners Brand.
New Products
In March 2021, Adjupharm launched a new medical cannabis inflorescence product with THC and CBD levels of 17% and 1%, respectively, under the IMC brand. The new product will be distributed pursuant to Adjupharm's distribution agreements with its German distribution partners.
In March 2021, Adjupharm entered into a supply agreement with MediPharm Labs, which will enable Adjupharm, subject to the fulfilment of applicable regulatory and import requirements, to launch a new category of IMC-branded extracts in Germany. This will include a range of specially formulated high THC, balanced THC and CBD cannabis oil products expected to launch in Germany in the second half of 2021.
The Group intends to, subject to applicable laws and regulatory approvals, distribute CannEpil®, a phytocannabinoid medicine developed by MGC, to be used for the treatment of refractory epilepsy. The distribution of CannEpil® is subject to the execution of a definitive distribution agreement.
7 (1) The actual percentages of THC and CBD content are determined by certified laboratory inspections and disclosed on the label of each IMC-branded medical cannabis product sold in Israel. Depending on such THC and CBD content, each IMC-branded medical cannabis product is labelled based on the following categories, in accordance with MOH Regulations: (a) 'T20/C4' (THC 16-24% and CBD 0-7%); (b) 'T15/C3' (THC 11-19% and CBD 0-5.5%); (c) 'T10/C2' (THC 6-14% and CBD 0-3.8%); (d) 'T10/C10' (THC 6-14% and CBD 6-14%); (e) 'T5/C5' (THC 1-9% and CBD 1-9%); (f) 'T0/C24' (THC 0-0.5% and CBD 20-28%); (g) 'T1/C20' (THC 0-2.5% and CBD 16-24%); (h) 'T3/C15' (THC 0.5-5.5% and CBD 11-19%); and (i) 'T5/C10' (THC 2.5-7.5% and CBD 6-14%). For all IMC-branded medical cannabis products, CBN content is less than or equal to 1.5%. The stated THC, CBD and CBN percentage ranges for the IMC branded strains are expected ranges; the actual percentages, as labelled on product packaging under the IMC brand, may vary or deviate from such ranges.
8 An independent clinical survey evaluated the effect of IMC branded strains on 652 Israeli medical cannabis patients licensed by the MOH for consuming medical cannabis products. The survey was managed, to the request of the Company, by an independent CRO - MediCaNL Israel, with data collected by third party survey and polling company iPanel, and analyzed by Dr. Nira Morag, senior lecturer, biostatistics department, Tel Aviv University.
- 24 -
The Group intends to, subject to applicable laws and regulatory approvals, distribute CannEpil®, a phytocannabinoid medicine developed by MGC, to be used for the treatment of refractory epilepsy. The distribution of CannEpil® is subject to the execution of a definitive distribution agreement.
Revenue
The following table shows the sales figures in dollars for each category of products that accounted for 15% or more of the total consolidated revenue of the Company for the financial years ended December 31, 2020 and 2019, derived from (a) sales to entities in which the Company maintains an investment accounted for by the equity method; (b) sales to customers, other than those referred to in (a); and (c) sales or transfers to controlling shareholders.
|
Revenue By Product Type |
||||
|
Financial Year |
Medical Cannabis |
Medical Cannabis |
Other |
Total |
|
2020 |
$4,673,000 |
$8,697,000 |
$2,520,000 |
$15,890,000 |
|
2019 |
$2,907,000 |
$3,405,000 |
$2,762,000 |
$9,074,000 |
Notes:
(1) IMC-branded medical cannabis products marked and sold under the category 'T15/C3', reflecting THC content of 11-19% and CBD content of 0-5.5%.
(2) IMC-branded medical cannabis products marked and sold under the category 'T20/C4', reflecting THC content of 16-24% and CBD content of 0-7%.
Production, Distribution and Sales in Principal Markets
Israel
The Company does not directly produce or distribute medical cannabis products in Israel. Pursuant to the Commercial Agreements, Focus propagates and cultivates medical cannabis products to be distributed under the IMC brand. Finished medical cannabis products are sold by Focus under the IMC brand to local pharmacies in Israel through contracted distributors. Focus holds a license from the MOH to propagate and cultivate medical cannabis in the State of Israel (the "Focus License"). Focus is one of the eight medical cannabis producers initially licensed by Israeli regulatory authorities and has over 10 years of experience in growing high quality medical cannabis products for the Israeli market. The MOH recently renewed the Focus License to be valid until January 3, 2022. All of Focus' operations are performed pursuant to the Dangerous Drugs Ordinance and the related regulations issued by IMCA.
Focus currently operates the Focus Facility, which has a total of approximately 300,000 square feet of cultivation capacity and a current annual output capability of up to 5,000kg of medical cannabis. Focus supplements its cultivation and production output by securing supply agreements with third-party cultivators to deliver medical cannabis for sale under the IMC brand.
- 25 -
The Company is actively seeking to provide its intellectual property, know-how and services to other Israeli medical cannabis producers.
Europe
The Company replicated its Israeli business strategy and established its medical cannabis brand in the European market through Adjupharm, a certified EU-GMP distributor in Germany with wholesale, narcotics handling, manufacturing, procurement, storage and distribution licenses granted by German regulatory authorities that allow for import/export capability with requisite permits (the "Adjupharm Licenses"). Adjupharm serves as the Company's flagship European outpost for sales and distribution.
Adjupharm currently manufactures and distributes IMC-branded medical cannabis products, in addition to other branded medical cannabis products, to pharmacies and distribution partners in Germany pursuant to sales and distribution agreements. Similar to Focus, Adjupharm sources its medical cannabis products from strategic partners, including various pan-European EU-GMP suppliers. While the Company does not currently distribute products in other European countries other than in Germany, the Company intends to leverage the platform established by Adjupharm in Germany and its network of distribution partners to expand to other jurisdictions across the continent in which medical cannabis is legal.
Germany
The Company's European strategy is centered in Germany, whose medical cannabis market is currently considered the largest in Europe.9 Adjupharm serves as the Company's principal operating hub in the German market and was originally acquired by IMC Holdings in early 2019. The Company, through IMC Holdings, currently owns 90.02% of Adjupharm, with the balance owned by Adjupharm's CEO.
The Company continues to develop Adjupharm as its European hub and to expand its presence in the German market by forging partnerships with pharmacies and distributors across the country. Led by Adjupharm's CEO Richard Balla, the Company's objective is to capture a significant market share in Germany by working directly with distributors to increase market reach for products bearing the IMC brand. The Company currently has an approximately 3,200 square feet EU-GMP production and warehousing facility in Germany and is in the process of expanding its facilities by an additional 3,200 square feet. Adjupharm sources its supply of medical cannabis for the German market from EU-GMP certified suppliers.
Adjupharm relies on its sales and distribution agreements to supply and distribute IMC-branded products to distribution partners in Germany, which are then distributed to German pharmacies. There are approximately 19,000 community pharmacies in Germany, each of which is permitted to create and dispense medications, including medical cannabis, pursuant to physician prescriptions.10 Adjupharm recently completed the expansion of its internal and external sales department and is focused on increasing physician awareness and engagement to drive sales of IMC-branded medical cannabis products. The competitive advantage in Germany lies in the Group's track record and brand reputation in Israel and proprietary data supporting the effectiveness of medical cannabis for the treatment of a variety of conditions.
9 Health Europa, June 23, 2020. https://www.healtheuropa.eu/exploring-growth-in-the-european-medical-cannabis-market/100849/
10 Federal Union of German Associations of Pharmacists: Figures Data Facts 2020.
- 26 -
The Company is actively seeking additional supply partners to diversify its sources of supply of premium and super premium cannabis products and further develop its European presence. For more details on Adjupharm's agreements with third-party distributors, see "General Development of the Business - Recent Developments".
Canada
Commencing March 18, 2021, following completion of the Trichome Transaction, the Company is acting in the adult-use recreational cannabis market in Canada, through Trichome, and TJAC, Trichome's wholly owned subsidiary. TJAC holds Standard Processing, Standard Cultivation and Sale for Medical Purposes licenses (collectively the "TJAC Licenses") issued to it by Health Canada, which are permitting it to cultivate, produce and sell cannabis products in Canada. Trichome was formed in 2017 as a specialty finance company focused on providing flexible and creative capital solutions to the global legal cannabis market, due to the lack of credit availability in the industry, extremely high equity valuations, and lack of business and industry maturity. From 2018 to 2020, Trichome provided secured financing to numerous companies in the cannabis sector, including licensed producers and retail operators. This strategy provided attractive rates of return on invested capital with downside protection given Trichome's high underwriting standards and the unique transaction structures employed.
Trichome is currently focused on supporting TJAC's operations and growing the related cannabis production platform by acquiring and restructuring cannabis assets that are complimentary to, and synergistic with TJAC.
TJAC operates an approximately 117,000 square feet indoor cultivation facility (the "Indoor Facility"), with approximately 80,000 square feet of space for the cultivation of cannabis. All of TJAC's cultivation occurs at this site, as well as certain processing activities, such as plant drying, bucking and trimming. Current cultivation capacity at the Indoor Facility is approximately 7,000kg per year, with the potential for approximately twice the amount of production in the next 12-24 months, subject to capital investment and procedural optimization. In addition, TJAC operates another facility of an approximately 15,000 square feet, which is the site where certain processing and all packaging, sales and shipping activities of the business occur (collectively with the Indoor Facility, the "TJAC Facilities"). The TJAC Facilities are located in in Kitchener, Ontario and are operated pursuant to certain lease agreements (the "TJAC Leases").
Like all licensed producers, TJAC is required to sell and distribute its products directly and only to the provinces of Ontario, British Columbia and New Brunswick, from which retail operators purchase their inventory. Licensed producers are not permitted to sell directly to retailer locations in such provinces. In Saskatchewan, TJAC sells its product to a third-party, intermediary wholesaler who then sells and distributes TJAC's product to the province's retail stores. In addition, TJAC has authorization to sell into Prince Edward Island and expects to do so in the coming months. TJAC is also in the process of applying for authorizations to distribute its cannabis portfolio in Alberta and Manitoba and is exploring partnership arrangements to facilitate the distribution of its products in Quebec through an authorized third-party licensed producer.
- 27 -
From time to time, TJAC has entered and may again enter into one-time, business-to-business purchase or supply agreements with other licensed producers, as may be required to back-fill its own purchase orders from the provinces (in the case of a purchase agreement) or back-fill another licensed producer's supply requirements (in the case of a supply agreement).
TJAC is in the late stages of winding up its medical sales program and will no longer fulfill sales directly to medical patients, effective as of May 1, 2021.
Specialized Skill and Knowledge
The Group relies on the expertise of its personnel to provide value to its clients. The Group has over 10 years of experience in cultivating, propagating and processing cannabis under the guidance of experienced master grower, Doron Reznik. Following the IMC Restructuring, IMC Holdings retained its master grower to continue providing cannabis cultivation and production advice exclusively to Focus and Focus' third-party cultivation partners.
Competitive Conditions
The medical and recreational cannabis industry in which the Group operates is, and is expected to remain, very competitive. Cannabis companies compete primarily on a regional basis, and competition may vary significantly from region to region at any particular time. The cannabis sector is in a high growth phase, with market participants engaged in significant expansion across global legal jurisdictions. The Company is working to achieve a leadership position in the cannabis industry by taking advantage of IMC brand recognition, earning superior margins as a fully integrated business, and leveraging its vast know-how and experience.
The Company faces competition in Israel among similar intellectual property-related service providers and from other established brands in the domestic market. The Company expects that its experience and track record, attained via the combination of Focus' operations over the past decade and IMC brand recognition, will distinguish its offerings from competitors in the Israeli market. Focus also competes with other licensed cultivators and purveyors of medical cannabis brands offering products to local pharmacies.
The Company's European operations will face competition from other entities licensed to cultivate, produce and distribute medical cannabis products in each respective jurisdiction. In Germany, Adjupharm will compete with a number of licensed distributors including currently established entities, expected new market entrants, and domestic producers of cannabis. Competitors vary from well-capitalized businesses with substantial operations and revenues to smaller or newer market entrants.
Components
The Group's ability to operate the business is dependent on its ability to source raw materials, skilled labour, and equipment from its supply partners around the world. In particular, required production inputs include but are not limited to biological assets, utilities, product packaging, and specialized equipment for propagating and cultivating cannabis. Although the Group does not foresee an issue with the availability of these inputs as needed, the Group is wary of any increases in pricing for such inputs. If prices of inputs were to significantly increase, this may cause a material adverse effect on the Group's business operations and financial condition. See "Risk Factors - Reliance on Key Business Inputs" below for additional details.
- 28 -
Intangible Properties
The Company relies on the licensing of its brand in Israel to widen its reach and offer branding, marketing and other related services to participants in the Israeli medical cannabis industry. The Group also plans to rely on the IMC brand to facilitate the distribution of cannabis products in international markets. The Group owns trademarks and trade secrets that allow it to serve a range of cannabis industry participants.
"IMC" is a registered trade name and trademark valid in Israel through May 2027 and in Germany through May 2030. During the fourth quarter of 2020, the Company applied for the registration of "IMC" as a trade name and trademark with the European Union Intellectual Property Office including an extension through the World Intellectual Property Organization to Switzerland, Norway and the United Kingdom. In Canada, the Company has engaged with authorities regarding a trademark registered under the IMC name for use in connection with various food supplements, vitamins, minerals and proteins and is awaiting a response to its submissions.
In addition, TJAC has commenced trademark applications for the names "Wagners" and "Well Made Weed" in Canada. All intellectual property acquired by TJAC from its acquisition of substantially all of the assets of James E. Wagner Cultivation Corp. has been determined to be of no value to the company. Accordingly, TJAC has either abandoned or will be abandoning such intellectual property and/or related applications.
Cycles
The demand for both medical cannabis products and recreational cannabis products is not materially influenced by seasonal or cyclical trends , and subject to the continued increase in demand in the medical cannabis market,11 is consistent year-round. In addition, the Company's cultivation strategy, as well as its ability to secure additional supply from its cultivation partners, allows Focus, Adjupharm and TJAC to provide consistent supply of cannabis products to their respective customers all year-round.
Economic Dependence
The Company is substantially dependent on Focus' sales and distribution agreements with pharmacies and distributors in the Israeli market, as listed and described under "General Development of the Business - Recent Developments", and additional sales to pharmacies and distributors through purchase orders received from time to time, in order to maintain revenues. In light of this dependence, any failure to maintain the Focus License or the Focus Lease Agreement or keep the Focus Facility in good standing, could have a material adverse effect on the Group. For additional information on potential risks arising from the Company's dependence on Focus' operations, see "Risk Factors" below.
Focus relies on and is substantially dependent on supply agreements with third-party cannabis cultivators to fulfill the supply requirements of its distribution and sales agreements with pharmacies in the Israeli market. For further information on such supply agreements, see "General Development of the Business - Recent Developments".
11 IMCA - Up-to-date data from patient licenses (November 2020). P. 2 [Hebrew]
https://www.health.gov.il/Subjects/cannabis/Documents/licenses-status-november-2020.pdf
https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=2010000801&pickMembers%5B0%5D=2.30&pickMembers%5B1%5D=3.1&cubeTimeFrame.startMonth=01&cubeTimeFrame.startYear=2020&cubeTimeFrame.endMonth=02&cubeTimeFrame.endYear=2021&referencePeriods=20200101%2C20210201
- 29 -
Similar to Focus, Adjupharm is substantially dependent on the supply, sales and distribution agreements with suppliers and German distributors, as listed and described under "General Development of the Business - Recent Developments". Certain German distributors have entered into significant binding purchase commitments with Adjupharm whereby Adjupharm is to supply the German distributors with medical cannabis products bearing the IMC brand for distribution across Europe. Any failure to maintain the Adjupharm License in good standing could have a material adverse effect on the Group. For additional information on potential risks arising from the Company's dependence on Adjupharm's operations, see "Risk Factors" below.
TJAC relies on and is substantially dependent on supply agreements for the sale of its products with certain provinces in Canada, with particular concentration in Ontario and British Columbia, in order to maintain revenues. A loss of one or a number of such supply agreements could materially impact TJAC's profit margins for the foreseeable future by requiring TJAC to sell at lower margins through alternative sales channels, unless more suitable sales arrangements could be secured. Furthermore, in light of this dependence, any failure to maintain the TJAC Licenses or the TJAC Leases in good standing could have a material adverse effect on the Group.
Changes to Contracts
There were no changes to contracts that materially affected the Company's financial year ended December 31, 2020. As of the date of this Annual Information Form, the Company does not anticipate being materially affected by the renegotiation or termination of any contracts or sub-contracts in the current financial year.
Environmental Protection
The Group's operations are subject to local environmental and safety laws and regulations concerning, among other things, emissions and discharges to water, air and land, the handling and disposal of hazardous and nonhazardous materials and wastes, and employee health and safety. The Company places great importance on being a constructive and responsible contributor to the environment and incurs ongoing costs and obligations to ensure compliance with all applicable environmental and employee health and safety matters.
Employees
As of December 31, 2020, the Company and its subsidiaries employed approximately 24 employees and Focus employed approximately 55 employees.
As of the date of this Annual Information Form, the Company and its subsidiaries employ approximately 200 employees and Focus employs approximately 50 employees. Medical Cannabis Regulatory Framework in Israel and Germany.
Medical Cannabis Regulatory Framework in Israel and Germany
To operate its business, the Company must abide by applicable medical cannabis laws in those countries in which it operates, namely Israel and Germany. Each jurisdiction has unique laws and regulations on the propagation, cultivation, production, distribution, use, import and export of medical cannabis products and the current regulatory frameworks continue to evolve. The Company cooperates with the regulatory authorities in those jurisdictions in which it operates to ensure that it is at all times in full compliance with applicable laws, rules and regulations.
- 30 -
Israel
Medical cannabis was first made available to patients in Israel in the early 1990s.12 Since then, Israel has developed one of the oldest medical marijuana research and business centers in the world, hosting dozens of cannabis companies and clinical studies pioneering how the plant can be used to treat cancer, epilepsy, post-traumatic stress disorder and other conditions. It has also been a leader in cultivation science.
The regulatory framework of medical cannabis in Israel has developed alongside the industry, as government organizations and directives have been established to legalize and facilitate the commercial operations of medical cannabis products in the country. In Israel, cannabis is currently defined as a "dangerous drug" according to both the Dangerous Drugs Ordinance13 and the 1961 Single Convention on Narcotic Drugs, to which Israel is a signatory. However, both the Dangerous Drugs Ordinance and the 1961 Single Convention on Narcotic Drugs allow for the use of cannabis for medical or research purposes under a supervised and controlled regime. In part to the growing evidence on the medicinal benefits of cannabis, the Israeli government on August 7, 2011 published Government Res. No. 306914 which laid the foundation for the National Medical Cannabis Program (the "NMCP"). The MOH, which is responsible for determining Israel's policies on matters of health and medical services, established the IMCA, or "YAKAR" in Hebrew, as the key agency to administrate the NMCP and the regulation of the Israeli cannabis sector. Regulated activities include the cultivation, production, distribution, delivery, possession, transportation, destruction of medical cannabis and related laboratory services. Israeli laws require anyone engaging in such activities to obtain an appropriate license issued by the IMCA under the Dangerous Drugs Ordinance and comply with the terms and conditions of such license. The production and distribution of adult-use recreational cannabis products is currently illegal in Israel.
The Israeli government has acknowledged that cannabis-based products may assist patients with certain medical conditions. In 2016, the Israeli government passed Government Res, No. 1587 ("Resolution 1587"),15 which outlines the "medicalization" of cannabis products. Resolution 1587 ensures the establishment of professional criteria for medical conditions to authorize patients for treatment with medical cannabis products, accessibility to the treatment, supply of medical-grade cannabis products and proper supervision of the product. Pursuant to the NMCP, the MOH is authorized to issue permits to patients related to the use of cannabis for medicinal purposes. The IMCA has established a regulatory "road map" for the licensing of commercial activities in the medical cannabis field, including the operation of commercial medical cannabis facilities in Israel.
The IMCA has since issued additional regulations setting out appropriate quality standards for the medical use of cannabis in a manner similar to the use of existing medicines. Israel has also begun establishing and broadening the scope of the NMCP to apply to all levels of the supply chain of medical cannabis based on Israeli government resolutions and administrative orders issued by the MOH. Activities conducted pursuant to the NMCP are under the control and supervision of the IMCA.
12 Ministry of Economy and Industry State of Israel, Israel's Medical Cannabis Innovation (August 2019). Pg. 12. https://investinisrael.gov.il/Documents/RoundTable/medical-cannabis-doc250919.pdf
13 Cannabis is listed in schedule 1 of the Dangerous Drugs Ordinance [New Version], 1973 [Hebrew]
https://www.health.gov.il/LegislationLibrary/Samim_01_EN.pdf
14 Israeli Government Res. No. 3609 [Hebrew], August 7th, 2011 https://www.gov.il/he/Departments/policies/2011_des3609
15 Israeli Government Res. No. 1587 [Hebrew], June 26, 2016 https://www.gov.il/he/departments/policies/2016_dec1587a
- 31 -
Patient Medical Use
The IMCA is responsible for reviewing applications and issuing permits to patients to hold and use medical cannabis products pursuant to Procedure 106 of the MOH ("Procedure 106").16 Procedure 106 sets out a list of medical conditions that may be treated with medical cannabis products. Such authorized medical conditions are examined and updated from time to time. For medical conditions that are not listed in Procedure 106, patients can apply for exceptions on extraordinary grounds.
An application for the approval of cannabis use for medical reasons, or an application for a license renewal or a change of dosage or form of consumption, must be submitted according to Procedure 106.
According to data from the MOH17 from November 2020, there were 77,338 patients licensed for medical cannabis usage in Israel. This figure reflected an increase of approximately 50% from the MOH's November 2019 figures.
Licensing and Authorization for Commercial Activities in the Medical Cannabis Field
In December 2017, pursuant to the NMCP, the MOH issued regulations that standardized the licensing process of growers, manufacturers, suppliers and pharmacies wishing to conduct commercial activities in the field of medical cannabis (the "Road Map").18
Pursuant to the Road Map, each operation in the medical cannabis field, including the cultivation, production, distribution, delivery, possession, transportation, destruction, and laboratory services, requires compliance with the provisions of applicable laws, including the procurement of an appropriate license under the Dangerous Drug Ordinance from the IMCA and the maintenance of such license in good standing. In addition, the Road Map does not allow an operating entity to conduct operations in more than one of the 'growth', 'production' and 'delivery' sub-sectors of the commercial medical cannabis chain; however, owners may partially or wholly own multiple operating entities operating in different sub-sectors.
The following is a list of operational licenses that may be obtained pursuant to Israeli regulations (the "Cannabis Licenses", and each a "Cannabis License") which, subject to certain exceptions, must be held by separate legal entities:
License for a medical cannabis propagation facility*;
License for a medical cannabis cultivation facility*;
License for a medical cannabis products manufacturing facility;
License for a medical cannabis storage and retail facility;
16 Ministry of Health Pharmaceutical Division Policy Number 106 - Licenses for Use of Cannabis
https://www.health.gov.il/hozer/DR_106.pdf (in Hebrew)
17 Updated Data from Patients' Licenses, November 2020 - https://www.health.gov.il/Subjects/cannabis/Documents/licenses-status-november-2020.pdf (in Hebrew)
18 Directive 107 - Guidelines for the process of licensing the practice of cannabis for medical use, as amended on October 2020 [Hebrew] - https://www.health.gov.il/hozer/CN_107_2019.pdf
- 32 -
License for a pharmacy authorized to distribute medical cannabis; and
Other licenses for the destruction, transportation and research and development activities with respect to medical cannabis.
*These licenses may be held concurrently by a single entity.
Cannabis Licenses may not be transferred, exchanged or assigned. They are valid for a period of up to 3 years and may be renewed with the approval of the IMCA.
The MOH has issued a set of directives containing procedures and requirements for Cannabis License applicants and has authorized certain entities to issue official certificates upon compliance with such directives. These directives include (i) Directive 150 (GSP Standard certification); (ii) Directive 151 (GAP Standard certification); (iii) Directive 152 (GMP Standard certification); and (iv) Directive 153 (GDP Standard certification).
Changes under the MOH Regulations
Until September 2019, patients licensed for consumption of medical cannabis products by the IMCA received all of their medical cannabis products authorized under their respective licenses at a fixed monthly price of NIS 370, regardless of each patient's authorized amount. As an example, a patient that was to receive 20 grams of medical cannabis products per month would pay the same monthly fee of NIS 370 as a patient that received 180 grams per month. In addition, IMCA assigned patients to a particular licensed medical cannabis producer, from which each patient would exclusively receive their medical cannabis products. Under the previous medical cannabis regulations, Focus distributed approximately 80% of its medical cannabis products via home delivery and the remaining 20% via an IMCA-established distribution outlet.
Under the MOH's new regulations, medical cannabis products are delivered from a licensed producer to a manufacturer, which then delivers to a distributor to distribute to pharmacies. In addition, patients licensed for consumption of medical cannabis products are no longer exclusively assigned to medical cannabis producers and may purchase medical cannabis products from authorized pharmacies at a range of price points without any MOH-regulated price controls.
In light of the MOH's new regulations, some medical cannabis patient licenses granted under the previous regime are still valid. The medical cannabis patient licenses set to expire during the period from February 1, 2019 to July 31, 2019 were extended by order of the Israeli Supreme Court until further notice by the Court. While these licenses remain valid, the patients that hold these licenses are entitled to receive medical cannabis products pursuant to the price controls and supplier restrictions of the former regime. Additional information on the proceedings pursuant to which the above-referenced order was granted can be found under "Legal Proceedings and Regulatory Actions - Legal Proceedings - Supreme Court of Justice 2335/19".
Medical Cannabis Imports
In October 2020, the MOH issued an updated procedure, titled "Guidelines for Approval of Applications for Importation of Dangerous Drug of Cannabis Type for Medical Use and for Research" ("Procedure 109"), describing the application requirements for cannabis import licenses for medical and research purposes. According to Procedure 109, the following permits and licenses are required to receive a cannabis import license:
1. License to possess medical cannabis and operate in the medical cannabis industry;
- 33 -
2. License to import plant material;
3. Permit to import narcotic drugs; and
4. License to import a dangerous drug.
Medical Cannabis Exports
The Israeli government approved a legislative reform on January 27, 201919 (the "Export Resolution") allowing the export of certain cannabis products, subject to the terms and conditions of the applicable license granted by the MOH. In addition, the cannabis products must meet the quality standards of the MOH and be delivered only to countries that have signed the 1961 Single Convention on Narcotic Drugs20 and approved the import of cannabis products into their territory; provided however, that the export shall be made, and the applicable export license shall be provided, in accordance with the respective regulations set by the MOH.
In October 2020, the MOH launched a new pilot program under which medical cannabis producers would be authorized to export medical cannabis products, subject to the requirement that certain products be made available at a fixed price of NIS 14 per gram to patients in Israel over the age of 21 and NIS 10 per gram to patients under the age of 21 (the "Pilot Program"). Each participating company would decide the selection of medical cannabis products made available under the Pilot Program. The Pilot Program was planned for an initial period of three months and was extended in January 2021. As products bearing the IMC brand are offered as part of the Pilot Program, IMC-branded products are eligible for immediate application for export permits.
In December 2020, the IMCA published guidelines for the medical cannabis export permit application process21 (the "Export Guidelines"), pursuant to which an export permit will only be granted to an applicant if (i) sufficient domestic supply has been secured by such applicant in the variety and quantity that will meet the Israeli level of demand; (ii) the delivery of medical cannabis is made from approved sites; (iii) the applicant has a valid IMC-GDP certification and business license from the IMCA; and (iv) an import permit from the importing country is obtained and attached to the export application. The term to apply for export permits under the program, according to the Export Guidelines, was set to expire at the end of Q1 2021. Further extensions are being considered by the IMCA based on the success of the Pilot Program.
Legalization of Adult-Use Recreational Cannabis in Israel
As of the date of this Annual Information Form, adult-use recreational cannabis use in Israel is illegal. In November 2020, an Israeli government committee responsible for advancing the cannabis market reform published a report supporting and recommending the legalization of adult-use recreational cannabis in Israel (the "Report"). Based on the Report, the Israeli Ministry of Justice was expected to formulate a bill to begin the legislative process towards the legalization of adult-use recreational cannabis. The government committee made its recommendation for legalization based on the increasing demand for adult-use recreational cannabis in Israel, the importance of maintaining quality standards and limiting uncontrolled products, the need for increased access to cannabis by medical patients and the objective of decreasing the size of the illegal market. The model proposed by the government committee in the Report is similar in nature to the model adopted in Canada, whereby the sale of adult-use recreational cannabis would be channeled through government-licensed dispensaries.
19 Directive 4490 [Hebrew] - https://www.gov.il/he/departments/policies/dec4490_2019
20 Single Convention on Narcotic Drugs, 1961, as amended by the 1972 Protocol amending the Single Convention on Narcotic Drugs, 1961 - https://www.unodc.org/pdf/convention_1961_en.pdf
21 Directive 110, December 2020 [Hebrew] - https://www.health.gov.il/hozer/CN_110.pdf
- 34 -
In December 2020, the governing Israeli parliament dissolved and general elections were scheduled for March 2021. All such legislative initiatives were suspended and there is no certainty regarding their renewal following a formation of a new government pursuant to the March 2021 elections.
Germany
On March 10, 2017, the German federal government enacted bill Bundestag-Drucksache 18/8965 - Law amending narcotics and other regulations that amended existing narcotics legislation to recognize cannabis as a form of medicine (in concrete: narcotic), and allow for the importation and domestic cultivation of medical cannabis products. Under the updated legislation, cannabis is listed in Annex 3 to the Federal Narcotics Act ("BtMG") as a "marketable narcotic suitable for prescription". Legalization in Germany applies only to cannabis "stemming from cultivation for medicinal purposes under state control in accordance with Articles 23 and 28 (1) of the 1961 Single Convention on Narcotic Drugs, and in preparations as finished medicinal products." Currently, the production, distribution, exportation and importation of medical cannabis products in Germany is legal, subject to regulations and licensing requirements, while operations involving adult-use recreational cannabis products remain illegal. Medical cannabis in Germany must comply with the corresponding monographs of the German and European pharmacopoeia.
The German medical cannabis licensing regime can be separated into two parts: generally, on the basis of Section 13 of the German Medicines Act ("AMG"); and with regard to narcotics, on the basis of Section 3 of the BtMG (both under federal jurisdiction). The import, export and distribution of medical cannabis currently requires a wholesale permit pursuant to Section 52a of the AMG and a distribution permit for narcotics pursuant to Section 3 of the BtMG. Manufacturing operations require authorizations pursuant to Sections 13 and 52a of the AMG. All BtMG permit applications must specify the strains and estimated quantities of medical cannabis involved and any subsequent changes must be reported to the Federal Opium Agency of Germany. The import of medical cannabis from other EU and non-EU countries requires quantity-based import licenses pursuant to Section 11 of the BtMG. In addition, for imports from a non-EU country, an import certificate pursuant to Section 72a of the AMG is required and in certain circumstances, depending on the import source, a general import permit may also be required under Section 72 of the AMG.
Unlike cannabis, CBD is not subject to German narcotics laws and may or may not be subject to German drug laws, depending on its use and dosage. Annex 1 of the Ordinance on the Prescription of Medicinal Products stipulates that CBD is in principle subject to prescription but does not specify a minimum quantity or a specific dosage form. However, a distinction must be made between consumable products that naturally contain CBD and those that are infused with CBD extract; the European Commission considers the latter to be a type of "food". In light of the above, various products containing CBD can be found in the German market.
Cultivation in Germany and Distribution of Medical Cannabis Cultivated in Germany
The Federal Opium Agency of Germany's Federal Institute for Drugs and Medical Devices ("BfArM") formed a cannabis division (the "Cannabis Agency") to oversee cultivation, harvesting, processing, quality control, storage, packaging and distribution to wholesalers, pharmacists and manufacturers. The Cannabis Agency also regulates pricing of German-produced medical cannabis products and serves as an intermediary of medical cannabis product sales between manufacturers, wholesalers and pharmacies on a non-profit basis.22 The Cannabis Agency has no influence on the actual retail price of medical cannabis products. The responsibilities of the Cannabis Agency are based on the requirements of the 1961 Single Convention on Narcotic Drugs. The Cannabis Agency is not responsible for the import of medical cannabis products and will therefore neither purchase nor distribute imported medical cannabis products. As a wholesaler, the Cannabis Agency sells German-based medical cannabis products in its own name. The Cannabis Agency contracted with a distributor that was selected in a Europe-wide tender procedure and commissioned it to carry out the distribution of medical cannabis products in accordance with all pharmaceutical and narcotic legal requirements.
22 www.bfarm.de/DE/Bundesopiumstelle/Cannabis/Cannabisagentur/_node.html
- 35 -
In late 2018, the Cannabis Agency issued a call for tenders to award licenses for local medical cannabis cultivation and distribution of German-cultivated medical cannabis products (the "German Local Tender"). The Cannabis Agency would serve as an intermediary in the supply chain between such cultivation and distribution. Following a delay caused by a legal proceeding regarding the initial tender process, BfArM relaunched the application process and selected 13 cultivation lots in April 2019 to receive licenses. Each license permitted the holder to grow up to 200kg per year for total production of 2,600kg per year collectively from the 13 cultivation lots and 10,400kg over the four-year license period. According to the German government, the first deliveries of medical cannabis products for purchase by the Cannabis Agency are expected in the first quarter of 2021.23 As no further notifications have been published in this regard, it cannot be assumed that harvesting took place in the first quarter. Rather, it can be assumed that one will only take place in the course of 2021. For distribution of locally cultivated medical cannabis products, one pharmaceutical wholesaler was granted a distribution license in order to organize the storage and distribution of medical cannabis products to pharmacies on behalf of the Cannabis Agency.
Import volumes and procedures
The current regime permits the importation of cannabis plants and plant parts for medicinal purposes under state control subject to the requirements under the 1961 Single Convention on Narcotic Drugs. Pursuant to the 1961 Single Convention on Narcotic Drugs, Germany must estimate the expected demand of medical cannabis products for medical and research purposes for the following year and report such estimates to the International Narcotics Control Board. The estimates are also required to be reported by the Federal Opium Agency of Germany by June 30th of each year.
As a prerequisite to obtaining a German import license, an applicant must have EU-GMP Standard and EU-GACP Standard certifications. All medical cannabis products imported to Germany must have been cultivated in a country with regulations compliant with Articles 23 and 28(1) of the 1961 Single Convention on Narcotic Drugs, and must comply with the relevant monographs described in the German and European pharmacopeias. While these requirements also apply to the exportation of medical cannabis products, the current German regime does not allow domestically cultivated medical cannabis products to be directly sold to commercial entities other than the Cannabis Agency.
Dispensing Exclusively via Pharmacies
Medical cannabis products imported pursuant to an import license under the BtMG and AMG/BtMG permits are sold exclusively to pharmacies for final dispensing to patients on a prescription basis as 'magistral preparations', a term used in Europe to refer to medical products prepared in a pharmacy in accordance to a medical prescription for an individual patient. Magistral preparations require certain manufacturing steps in the pharmacy. Such manufacturing steps of the pharmacist typically include the testing and dosing of pre-packaged cannabis inflorescences (typically referred to as "floss"), medical cannabis products for oral administration (dronabinol), medical cannabis products for inhalation upon evaporation, and medical cannabis-infused teas.
23 www.bfarm.de/SharedDocs/Pressemitteilungen/DE/2019/pm4-2019.html
- 36 -
In addition to magistral preparations, medical cannabis products are also marketable as pre-packaged, licensed drugs.
Recreational Cannabis Regulatory Framework in Canada
The Cannabis Act and the Cannabis Regulations (Canada) made thereunder (the "Cannabis Regulations") came into force on October 17, 2018, legalizing the sale of adult-use recreational cannabis. The Cannabis Act and Cannabis Regulations establish a licensing and permitting scheme for the production, importation, exportation, testing, packaging, labelling, sending, delivery, transportation, sale, possession and disposal of adult-use recreational cannabis.
On October 17, 2019, amending regulations titled the Regulations Amending the Cannabis Regulations came into force that, among other things, expanded the scope of the Cannabis Act and Cannabis Regulations to enable the sale of certain categories of cannabis, including cannabis extracts, topicals and edibles, and set THC content limits for certain categories of cannabis products.
Licensing
The Cannabis Regulations establish six classes of licenses under the Cannabis Act: (i) cultivation licences, including standard cultivation, micro-cultivation and nursery sub-classes; (ii) processing licences, including standard processing and micro-processing sub-classes; (iii) analytical testing licences; (iv) sales for medical purposes licences; (v) research licences; and (vi) cannabis drug licences. These licences are valid for a period of up to five years. Licence requirements and rules differ depending on the class and/or sub-class of the licence.
Security Clearances
Certain people associated with cannabis licensees must hold a valid security clearance issued by Canada's Minister of Health. For example, in the case of corporations that hold licences for cultivation, processing or sale, directors, officers and other individuals who exercise, or are in positions to exercise, direct control over the corporation are required to hold such a security clearance. Under the Cannabis Regulations, the Minister may refuse to grant security clearances to individuals with organized crime associations or past convictions for, or in association with, drug trafficking, corruption or violent offences. Individuals who have a history of nonviolent, lower-risk criminal activity (for example, simple possession of cannabis or small-scale cultivation of cannabis plants) are not precluded by legislation from participating in the legal cannabis industry, and the granting of security clearance to such individuals is at the discretion of the Minister.
Cannabis Tracking System
Under the Cannabis Act, the Minister is authorized to establish and maintain a national cannabis tracking system. Accordingly, Health Canada introduced the Cannabis Tracking and Licensing System, whereby licence holders are required to use this online system to submit monthly tracking reports, new license applications and licence renewal requests, among other things. The purpose of this system is to track cannabis throughout the supply chain to help prevent diversion of cannabis into, and out of, the legal market. The Cannabis Act provides the Minister with the authority to make a ministerial order that would require licensees to report specific information about their authorized activities with cannabis, in the form and manner specified by the Minister.
- 37 -
Cannabis Products
The Cannabis Act and Cannabis Regulations, as amended, set out the requirements for the sale of dried cannabis, fresh cannabis, cannabis plants, cannabis seeds, cannabis edibles, cannabis extracts and cannabis topicals. Among other requirements, THC content limits are prescribed depending on the product category.
Packaging & Labelling
The Cannabis Regulations set out detailed requirements pertaining to the packaging and labelling of cannabis products that seek to promote informed consumer choice and allow for the safe handling and transportation of cannabis, while also reducing the appeal of cannabis to youth and promoting safe consumption. These requirements include plain packaging for cannabis products and packaging that is tamper-proof and child-resistant. The Cannabis Regulations further require package labels to include, among other information, the class of cannabis and the name, phone number and email of the licensed cultivator or processor, the standardized cannabis symbol and information pertaining to the THC and CBD content. Specific requirements vary depending on the product category of cannabis.
Promotion
The Cannabis Act prohibits the promotion of cannabis, cannabis accessories and cannabis-related services unless authorized by the Cannabis Act through certain exceptions prescribed in the Cannabis Act and the Cannabis Regulations.
Medical Cannabis
In addition to governance of recreational cannabis activities, the Cannabis Regulations also govern the regulatory framework associated with medical cannabis in Canada. Prior to the coming into force of the Cannabis Act and Cannabis Regulations, the sale of medical cannabis was permitted under the ACMPR. Although the ACMPR was replaced by the Cannabis Act and Cannabis Regulations, the new rules were not significantly different from the previous rules; changes were made to improve patient access, ensure consistency with recreational cannabis rules, and reduce the risk of abuse within the medical access system.
Provincial and Territorial Regulatory Framework
While the Cannabis Act provides for the regulation of adult-use cannabis production by the federal government, provincial and territorial governments maintain authority to regulate other aspects of adult-use recreational cannabis activities such as sale and distribution, minimum age requirements, and places where cannabis can be consumed.
- 38 -
The following chart summarizes the basic recreational cannabis regimes in place as of the date of this Annual Information Form:
|
Province or Territory |
Minimum Age to |
Private and/or Public |
Online Sales |
|
Alberta |
18 |
Private and Public |
Yes (Public only) |
|
British Columbia |
19 |
Private and Public |
Yes (Public only) |
|
Manitoba |
19 |
Private |
Yes |
|
New Brunswick |
19 |
Public |
Yes |
|
Newfoundland and Labrador |
19 |
Private and Public |
Yes (Public only) |
|
Nova Scotia |
19 |
Public |
Yes |
|
Ontario |
19 |
Private and Public |
Yes (Public only) |
|
Prince Edward Island |
19 |
Public |
Yes |
|
Quebec |
21 |
Public |
Yes |
|
Saskatchewan |
19 |
Private |
Yes |
|
Northwest Territories |
19 |
Private and Public |
Yes (Public only) |
|
Nunavut |
19 |
Private and Public |
Yes |
|
Yukon |
19 |
Private and Public |
Yes (Public only) |
Bankruptcy
No voluntary or involuntary bankruptcy, receivership or similar proceedings have been engaged against Focus, the Company or any of the Company's subsidiaries during the financial years ended December 31, 2020, December 31, 2019, April 30, 2019. No proceedings have been engaged against Focus, the Company or any of the Company's subsidiaries as of the date of this Annual Information Form.
Reorganizations
Other than as described below, no material reorganizations of the Company or any of its subsidiaries have taken place within the financial years ended December 31, 2020, December 31, 2019, April 30, 2019, or as of the date of this Annual Information Form, and there are no proposed reorganizations as of the date of this Annual Information Form.
On October 11, 2019, the Company completed the Reverse Takeover Transaction. The Reverse Takeover Transaction was effected by way of a "triangular merger" between the Company, IMC Holdings and a wholly-owned subsidiary of the Company pursuant to Israeli statutory law. The Board and management of the Company were reconstituted and subsequently led by Oren Shuster. As a result of the Reverse Takeover Transaction, the Company changed its business from mining to the international medical cannabis industry. Upon the completion of the Trichome Transaction, the Company expanded its activities to the Canadian recreational cannabis industry.
- 39 -
Social or Environmental Policies
The Company has not implemented any specific social or environmental policies that are fundamental to the Company's operations, other than as stated below. However, the Company consults with local advisors to ensure that the Group is in compliance with local environmental laws in each of the Group's operational jurisdictions.
TJAC has drafted and implemented very strict health and safety policies governing employee operations regarding hearing protection, eye protection and respiratory protection that supersedes minimum levels suggested by the Canadian Ministry of Labour.
RISK FACTORS
There are certain risks associated with owning securities of the Company that holders should carefully consider. The risks and uncertainties below are not the only risks and uncertainties facing the Company. Additional risks and uncertainties not presently known to the Company or that the Company currently considers immaterial may also impair the business, operations and future prospects of the Company and cause the price of its securities to decline. If any of the following risks actually occur, the business of the Company may be harmed and its financial condition and results of operations may suffer significantly. In that event, the trading price of the Company's securities could decline, and holders may lose all or part of their investment. In addition to the risks described elsewhere in the Company's filings on SEDAR at www.sedar.com, holders of securities should carefully consider each of, and the cumulative effect of all of, the following risk factors.
General Business Risk and Liability
Given the nature of the Company's business, it may from time to time be subject to claims or complaints from investors or others in the normal course of business. The legal risks facing the Company, its directors, officers, employees or agents in this respect include potential liability for violations of securities laws, breach of fiduciary duty or misuse of investors' funds. Violations of securities laws and breach of fiduciary duty could result in civil liability, fines, sanctions, or the suspension or revocation of the Company's right to carry on its existing business. The Company may incur significant costs in connection with such potential liabilities.
Consolidation of Focus Financial Results under IFRS 10 and Maintenance of Common Control
The Company complies with IFRS 10, which applies a single consolidation model using a definition of "control" that requires an investor (as defined in IFRS 10) to consolidate an investee (as defined in IFRS 10) where: (i) the investor has power over the investee; (ii) the investor has exposure or rights to variable returns from involvement with the investee; and (iii) the investor can use its power over the investee to affect the amount of the investor's returns.
- 40 -
Subsequent to the IMC Restructuring, the Company analyzed the terms of the contractual agreements with Focus (including the Commercial Agreements and the Focus Agreement) in accordance with IFRS 10 to conclude whether it should continue to consolidate the accounts of Focus in its financial statements.
Under IFRS 10, consolidation occurs when an investor can exercise control over an investee. Control is achieved through voting rights or other evidence of power. Where there are no direct holdings, under IFRS 10, an investor (as defined in IFRS 10) should consider other evidence of power and ability to unilaterally direct an investee's (as defined in IFRS 10) relevant activities. In view of the contractual agreements and the guidance in IFRS 10, notwithstanding that the Company has no direct or indirect ownership of Focus, it has sufficient rights to unilaterally direct the relevant activities (a concept known as "de facto control"), mainly due to the following:
(a) the Company receiving economic benefits from Focus (and the terms of the Commercial Agreements cannot be changed without the approval of the Company);
(b) the Company having the option to purchase the divested 74% interest in Focus held by Oren Shuster, the CEO, director and a promoter of the Company, and Rafael Gabay, a director and a promoter of the Company;
(c) Messrs. Shuster and Gabay each being a director of Focus (while Mr. Shuster concurrently being a director, officer and substantial shareholder of the Company and Mr. Gabay concurrently being a substantial shareholder of the Company); and
(d) the Company providing management and support activities to Focus through the Services Agreement.
Accordingly, under IFRS 10, the Company has "de facto control" over Focus, and therefore consolidates the financial results of Focus in the Company's financial statements.
Any failure of the Company or Messrs. Oren Shuster and Rafael Gabay to maintain "de facto control" over Focus as defined under IFRS 10 could alter the Company's consolidation model, potentially resulting in a material adverse effect on the business, results of operations and financial condition of the Company.
Possible Direct Involvement in the Israeli Cannabis Industry
Neither the Company nor any of its subsidiaries currently hold, directly or indirectly, any licenses to engage in the propagation, cultivation, production, processing, distribution or sale of medical cannabis products in Israel (the "Cannabis Activity"). According to current Israeli regulatory medical cannabis framework, any engagement in Cannabis Activity requires receiving the applicable license from the IMCA, which requires, among other things, pre-approvals by the IMCA of the directors, officers and shareholders holding over 5% of the license applicant and applies limitations on future securityholdings. Therefore, any direct engagement of the Company in Cannabis Activity will require the aforementioned approvals by the IMCA and will apply, upon such approval and granting of a license, limitations on future securityholdings. Furthermore, due to the uncertainty related to the broad administrative discretion over the activities in the medical cannabis industry in Israel granted to the IMCA, the aforementioned approvals and restrictions of the IMCA may apply to the Company and its shareholders by virtue of a subsidiary or investee engaging in Cannabis Activity.
Ownership of Focus
There is a risk that regulatory authorities in Israel may view the Company as the deemed owner of more than 5% of Focus and/or determine that the Company is in contravention of Israeli cannabis regulations. Namely, prior approval of the IMCA is required for any shareholder owning 5% or more of an Israeli company licensed to engage in cannabis-related activities. Any contravention of Israeli cannabis regulations could jeopardize the good standing of the Focus License. Such a determination may adversely affect the Group's ability to conduct sales and marketing activities and could have a material adverse effect on the Company's business, operating results or financial condition.
Limited Operating History
The Company did not generate revenue from the sale of cannabis products until late 2019. The Company is therefore subject to many of the risks common to early-stage enterprises, including under-capitalization, cash shortages, limitations with respect to personnel, financial, and other resources and lack of revenues. There is no assurance that the Company will be successful in achieving a return on shareholders' investment and the likelihood of success must be considered in light of the early stage of operations.
- 41 -
Negative Cash Flow from Operations
During the year ended December 31, 2020, the Company had negative cash flows from operating activities. Although the Company expects to generate positive cash flows from its future operating activities, there is no assurance that it will achieve this objective. If operational cash flows continue to be negative, the Company may be required to fund future operations with alternative financing options such as offerings of shares.
Additional Financing
There is no assurance that the Company will be able to secure the funds necessary to implement its strategies. Additional debt incurred by the Company from engagements such as major acquisitions may cause the Company's debt level to increase and result in difficulties in completing or negotiating future debt financings. Any triggering of credit defaults or failure to raise capital by the Company may cause significant delays in carrying out business objectives or result in a material adverse effect on the Company's business, financial condition, operational results and prospects.
Compliance with Laws
The Company's and its investees' operations are subject to various laws, regulations and guidelines. The Company endeavours to and cause its investees to comply with all relevant laws, regulations and guidelines. However, there is a risk that the Company's and its investees' interpretation of laws, regulations and guidelines, including, but not limited to the Cannabis Act, the regulations thereunder and applicable stock exchange rules and regulations, may differ from each other, and the Company's and its investees' operations may not be in compliance with such laws, regulations and guidelines. In addition, achievement of the Company's business objectives is contingent, in part, upon compliance with regulatory requirements enacted by governmental authorities and, where necessary, obtaining regulatory approvals. The impact of regulatory compliance regimes, any delays in obtaining, or failure to obtain regulatory approvals required by the Company or its investees may significantly delay or impact the development of the Company's business and operations and could have a material adverse effect on the business, results of operations and financial condition of Company. Any potential noncompliance could cause the business, financial condition and results of operations of Company to be adversely affected. Further, any amendment to or replacement of the Cannabis Act or other applicable rules and regulations governing the activities of the investees may cause adverse effects to Company's operations. The risks to the business of Company and its investees associated with the decision to amend or replace the Cannabis Act and subsequent regulatory changes, could reduce the addressable market for the Company's or the investees' products and could materially and adversely affect the business, financial condition and results of operations of the Company.
- 42 -
The Company and its investees incur ongoing costs and obligations related to regulatory compliance. Failure to comply with applicable laws and regulations may result in enforcement actions thereunder, including orders issued by regulatory or judicial authorities causing operations to cease or be curtailed, and may include corrective measures requiring capital expenditures or remedial actions. Parties may be liable for civil or criminal fines or penalties imposed for violations of applicable laws or regulations. Amendments to current laws, regulations and permitting requirements, or more stringent application of existing laws or regulations, may have a material adverse impact on the Company and/or its investees, resulting in increased capital expenditures or production costs, reduced levels of cannabis production or abandonment or delays in the development of facilities which could have a material adverse effect on the business, results of operations and financial condition of the Company.
The introduction of new tax laws, regulations or rules, or changes to, or differing interpretations of, or application of, existing tax laws, regulations or rules in any of the countries in which the Company invests could result in an increase in the Company's taxes, or other governmental charges, duties or impositions. No assurance can be given that new tax laws, regulations or rules will not be enacted or that existing tax laws, regulations or rules will not be changed, interpreted or applied in a manner which could result in the Company's profits being subject to additional taxation or which could otherwise have a material adverse effect on the Company.
Regulation of the Cannabis Industry
The cannabis-related business and activities of the Group are heavily regulated in all jurisdictions where it carries on business. The Group's operations are subject to various laws, regulations and guidelines by governmental authorities, particularly the MOH and the BfArM, relating to the manufacture, marketing, management, transportation, storage, sale, pricing and disposal of medical cannabis inflorescences and cannabis oil products. The Group's operations are also subject to laws and regulations relating to health and safety, insurance coverage, the conduct of operations and the protection of the environment.
The Group's operations may also be impacted by any future government regulation of adult-use recreational cannabis. As of the date of this Annual Information Form, an Israeli government committee responsible for advancing cannabis market reform has expressed positive views towards the legalization of adult-use recreational cannabis in Israel and the Israeli Ministry of Justice is expected to formulate a bill to allow for this objective. However, in December 2020, the governing Israeli parliament dissolved and general elections were scheduled for March 2021, suspending all such legislative initiatives including the legalization process for adult-use recreational cannabis. There is no certainty that any initiatives will be revisited following the formation of a new government pursuant to the March 2021 elections.
Notwithstanding the foregoing, the Group is well positioned to take advantage of the increased market opportunities provided by the legalization of adult-use recreational cannabis in Israel if it occurs. Any delays or abandonment to the legalization of adult-use recreational cannabis or changes to the political environment which would negatively affect the legalization of adult-use recreational cannabis in Israel may hinder demand and/or growth of demand for medical cannabis products bearing the IMC brand and may have material adverse effects to the Group's business.
- 43 -
Laws and regulations, applied generally, grant government agencies and self-regulatory bodies broad administrative discretion over the activities of the Group, including the power to limit or restrict business activities, including import and export of cannabis products as well as impose additional quality criteria an disclosure requirements on the Group's products and services. Achievement of the Group's business objectives are contingent, in part, upon compliance with regulatory requirements enacted by these governmental authorities and obtaining all regulatory approvals, where necessary, for the production and sale of its products.
The Company cannot predict the time required to secure all appropriate regulatory approvals for the Group's products and activity, or the extent of testing and documentation that may be required by governmental authorities. Any delays in obtaining, or failure to obtain regulatory approvals would significantly delay the development of markets and products and could have a material adverse effect on the business, results of operations, financial condition and prospects of the Group.
Failure to comply with the laws and regulations applicable to its operations may lead to possible sanctions including the revocation or imposition of additional conditions on licenses to operate the Group's business, the suspension or expulsion from a particular market or jurisdiction or of its key personnel, and the imposition of fines and censures. To the extent that there are changes to the existing laws and regulations or the enactment of future laws and regulations that affect the sale or offering of the Group's products or services in any way, this could have a material adverse effect on the business, results of operations, financial condition and prospects of the Group.
Changes in Laws, Regulations and Other Guidelines
The Group's operations are subject to a variety of laws, regulations, and guidelines relating to the marketing, acquisition, manufacture, management, distribution (including import and export), transportation, storage, sale and disposal of cannabis products. The Group's operations are also subject to laws and regulations relating to health and safety, insurance coverage, the conduct of operations and the protection of the environment. While the Group is currently in compliance with all such laws, regulations and guidelines, any rulings to the contrary or any changes to such laws and regulations that are beyond the control of the Group could have a material adverse effect on the business, results of operations, financial condition and prospects of the Group.
Environmental and Employee Health and Safety Regulations
The Group's operations are subject to environmental and occupational safety laws and regulations in certain jurisdictions, concerning, among other things, emissions and discharges to water, air and land, the handling and disposal of hazardous and nonhazardous materials and wastes, and employee health and safety. The Group incurs ongoing costs and obligations related to compliance with environmental and employee health and safety matters. Any failure to comply or maintain compliance with environmental and occupational safety laws and regulations may result in additional costs for corrective measures, penalties or restrictions on manufacturing operations. In addition, changes in environmental, employee health and safety or other laws, more vigorous enforcement thereof or other unanticipated events could require extensive changes to the Group's operations or give rise to material liabilities, which could have a material adverse effect on the business, results of operations and financial condition of the Group. This is particularly relevant for Focus and Adjupharm as these entities engage in cannabis-related operations that may be more prone to environmental and employee safety issues. Any changes to current laws and regulations may require substantial investments by the Group in order to comply such changes. If substantial investments are required, there may be a material adverse effect on the Group's operations, financial condition and operating results.
- 44 -
Reliance on License and Permit Renewals
Focus, Adjupharm and TJAC are dependent on the Focus License, Adjupharm Licenses and TJAC Licenses (together, the "Key Licenses"), respectively, and the need to maintain such Key Licenses in good standing. Failure to comply with the requirements or maintenance of any of the Key Licenses may have a material adverse effect on the business, financial condition and operating results of the Group. As of the date of this Annual Information Form, the Focus License is valid until January 3, 2022, the TJAC Licenses are valid until August 28, 2023, and the quantities for import under the Adjupharm Licenses are valid until May 8, 2021. Although management of Focus, Adjupharm and TJAC believe that they will continue to meet the requirements of the MOH, BfArM and Health Canada, respectively, for the respective durations of the Key Licenses, there can be no guarantee that the MOH, BfArM or Health Canada will extend or renew any of the Key Licenses or, if any of the Key Licenses are extended or renewed, that they will be extended or renewed on the same or similar terms.
Should the MOH, BfArM or Health Canada not extend or renew any of the Key Licenses, or should it renew any of the Key Licenses on different terms or not allow for anticipated capacity increases, the business, financial condition, results of the operations and prospects of the Group may subject to a material adverse effect.
Reliance on Other Business Licenses, Permits and Approvals
In addition to Focus' and Adjupharm's dependence on the Focus License and Adjupharm Licenses mentioned above, the Group is also dependent on ancillary business licenses, permits and approvals granted by government authorities or other third parties in order to operate effectively including, without limitation, building permits, municipal permits, third-party licenses, and foreign trade licenses. Should the Group fail to maintain any of these licenses, permits and approvals, or should it fail to renew any of such licenses, permits and approvals on materially similar or more favorable terms, the business, financial condition and results of the operations of the Group may be subject to a material adverse effect.
Reliance on Focus Facility
The Focus License is specific to the Focus Facility and both must remain in good standing for Focus to conduct the medical cannabis activities authorized thereunder. Adverse changes or developments affecting the Focus Facility, including but not limited to the failure to maintain all requisite regulatory and ancillary permits and licenses, the failure to comply with state or municipal regulations, or a breach of security, could have a material adverse effect on the Group's business, financial condition, results of operations and prospects.
In addition, any breach of the Focus Lease Agreement or any failure to renew the Focus Lease Agreement, on materially similar or more favorable terms, may have a material adverse effect on the Group's business, financial condition, results of operations and prospects, and could also have an impact on Focus' ability to continue operating under the Focus License or to renew the Focus License.
The Focus Facility is subject to state and municipal regulation and oversight, including the acquisition of all required regulatory and ancillary permits to conduct operations or undertake any construction. Any breach of regulatory requirements, security measures or other facility requirements, including any failure to comply with recommendations or requirements arising from inspections by government regulators at all levels, could also have an impact on Focus' ability to maintain the Focus Lease Agreement and/or keep the Focus Facility in good standing, and to continue operating under the Focus License or the prospect of renewing the Focus License.
- 45 -
The Focus Facility continues to operate with routine maintenance. Focus will bear many, if not all, of the costs of maintenance and upkeep of the Focus Facility, including replacement of components over time. Focus' operations and the Group's financial performance may be adversely affected if Focus is unable to keep up with maintenance requirements.
In December 2020, the municipal committee presiding over planning and construction in southern Israel (the "Construction Committee") advised Focus that it was the subject of certain allegations regarding inadequate permitting for construction relating to the Focus Facility (the "Construction Allegations"). Focus' shareholders and directors, including Oren Shuster and Rafael Gabay, received a summons and have testified before the Construction Committee. In January 2021, the MOH advised Focus that it had received a complaint of the same nature as the Construction Allegations (the "MOH Allegations"). Focus is fully cooperating with the ongoing investigations of both the Construction Committee and the MOH. As of the date of this Annual Information Form, no formal legal proceedings have been commenced against any of Focus, Mr. Shuster or Mr. Gabay. In the event that formal legal proceedings in respect of the Construction Allegations and/or the MOH Allegations are launched, potential consequences of any negative outcome may include, but are not limited to: (i) criminal charges against any or all of Focus or Focus' shareholders and directors, including Mr. Shuster and Mr. Gabay; (ii) monetary penalties or fines; (iii) temporary or permanent suspension of the Focus License; and (iv) other consequences that may limit, in part or as a whole, Focus' operations under the Focus License. A negative outcome to the Construction Allegations or the MOH Allegations may have a material adverse effect on the business, results of operations and financial conditions of the Group.
Reliance on the TJAC Leases
The TJAC Licenses are specific to the TJAC Facilities which are subject to the TJAC Leases and such licenses must remain in good standing for TJAC to conduct the cannabis cultivation, processing and sales activities authorized thereunder. Adverse changes or developments affecting the TJAC Leases, including but not limited to the failure to maintain all requisite regulatory and ancillary permits and licenses, the failure to comply with provincial or municipal regulations, or a breach of security, could have a material adverse effect on the Group's business, financial condition, results of operations and prospects.
In addition, any breach of either of the TJAC Leases or any failure to renew one or both of the TJAC Leases on materially similar or more favorable terms, may have a material adverse effect on the Group's business, financial condition, results of operations and prospects, and could also have an impact on TJAC's ability to continue operating under the TJAC Licenses or to renew any of the TJAC Licenses.
The TJAC Facilities are subject to the TJAC Leases and thus, subject to provincial and municipal regulation and oversight, including the acquisition of all required regulatory and ancillary permits to conduct operations or undertake any construction. Any breach of regulatory requirements, security measures or other facility requirements, including any failure to comply with recommendations or requirements arising from inspections by government regulators at all levels, could also have an impact on TJAC's ability to maintain the TJAC leases and/or keep TJAC Facilities in good standing, and to continue operating under the TJAC Licenses or the prospect of renewing one or both of the TJAC Licenses.
The TJAC Facilities continue to operate with routine maintenance. TJAC will bear some of the costs of maintenance and upkeep of the TJAC Facilities in accordance with the terms of the respective TJAC Leases, including replacement of components over time. TJAC's operations and the Group's financial performance may be adversely affected if TJAC is unable to keep up with maintenance requirements.
- 46 -
Product Security and Storage
The Group stores products in the Focus Facility and certain licensed Adjupharm facilities and the TJAC Facilities before delivering them to contracted parties. As part of the Israeli, German an Canadian licensing requirements, Focus, Adjupharm and TJAC are required to maintain certain standards of storage for cannabis products. The risk of inventory theft from these facilities is mitigated by Focus, Adjupharm and TJAC through the implementation of the security measures required under applicable laws, such as usage of qualified storage units, designated storage locations, locked storage vaults, access control, security cameras, and alert systems. Notwithstanding such security measures, any breaches of security may result in losses of inventory, potential litigation, and increased costs to bolster security.
Reliance on Key Suppliers
Focus and Adjupharm both rely on their respective supply agreements with cannabis cultivators and producers in order to meet the demands of their respective sales agreements with distribution partners and pharmacies. Consequently, the Group relies on the suppliers of such supply agreements to provide necessary cannabis products to Focus and Adjupharm. If any suppliers fail to supply any contracted materials to Focus or Adjupharm as a result of, among other things, disruptions related to major health issues or pandemics, or labor disputes, Focus or Adjupharm may fail to meet purchase commitments from their distribution partners. Any inability to secure required supplies and services or to do so on favourable terms could negatively impact the operations of Focus or Adjupharm. In addition, failures of suppliers to maintain required licenses, permits and approvals, including any import/export permits or comply with applicable laws, regulations and contractual specifications pertaining to the contracted cannabis products may also result in Focus' or Adjupharm's failure to meet purchase commitments. As the Group derives a significant portion of its revenue from the fulfilment of these purchase commitments, such supplier failures may lead to a material adverse effect on the business, results of operations and financial conditions of the Group.
Any product recalls, quality control issues with medical cannabis products supplied to Focus or Adjupharm, or inabilities of the Group to secure required supplies and services or to do so on adequate terms could also cause a material adverse effect on the Group's business, financial condition and results of operations.
Reliance on Key Distribution Partners
Focus and Adjupharm both rely heavily on their respective sales agreements with pharmacies and distribution partners in order to exchange payment for shipments of medical cannabis products pursuant to binding purchase commitments. Consequently, the Company relies on the distribution partners of such sales agreements to make payments as shipments of medical cannabis products are delivered. If any distribution partners fail to make delivery to pharmacies or payments to Focus or Adjupharm, the Company may experience a decline in the revenues it derives from Focus or Adjupharm. Such distribution partner failures may ultimately lead to a material adverse effect on the business, results of operations and financial conditions of the Group.
- 47 -
Reliance on Provincial Supply Agreements
TJAC relies heavily on its supply agreements for the sale of its products with certain provinces in Canada, with particular concentration in Ontario and British Columbia. A loss of one or a number of such supply agreements could materially impact TJAC's profit margins for the foreseeable future by requiring TJAC to sell at lower margins through alternative sales channels (such as business-to-business sales agreements with intermediary licensed producers), unless more suitable sales arrangements could be secured. A loss of one or more of TJAC's provincial supply agreements may ultimately lead to a material adverse effect on the business, results of operations and financial conditions of the Group.
Ability to Meet Target Production Capacity
Focus' sales agreements are subject to estimates in target production capacity at the time of such agreement. These estimates may prove to be inaccurate due to uncontrollable external factors such as genetic drifts in strain of plants grown and general difficulties in estimating growth of cannabis plants. Any adverse misalignments between the target production capacity and actual production capacity may result in a material adverse effect on the Company's business, financial condition and operating results.
Ability to Secure New Suppliers and Distribution Partners
The Group's success depends on its ability to secure suppliers and distribution partners. There are many factors which could impact the Group's ability to secure suppliers and distribution partners, including but not limited to IMC brand awareness, the Group's ability to continually produce desirable and effective cannabis products, compliance with regulatory requirements in connection with import and export of cannabis products, and the successful implementation of new partnership plans. The failure to secure suppliers or distribution partners could have a material adverse effect on the Company's business, financial condition, results of operations and prospects.
Reliance on Key Business Inputs
The Group's business is dependent on a number of key inputs and their related costs including raw materials and supplies related to its growing and distribution operations as well as electricity, water, and other utilities. Any significant interruption or negative change in the availability or economics of the supply chain for key inputs (e.g. rising energy costs) could cause a material adverse effect on the business, financial condition, and operating results of the Group. Any failure to secure required supplies and services or to do so on appropriate terms could also have a material adverse effect on the business, financial condition, and operating results of the Group.
Competition and Innovation to Achieve Strategic Objectives
In addition to being subject to general business risks applicable to a business involving an agricultural product and a regulated consumer product, the Group will need to make significant investments in its business strategy. These investments include the procurement of raw material, supplier and distributor outreach projects, and research and development projects. The Company expects that competitors will undertake similar investments to compete with it. Competitive conditions, third-party partner preferences, patient requirements and spending patterns in this industry and market are relatively unknown and may have unique circumstances that differ from other existing industries and markets and contribute to unsuccessful future business development or expansion efforts by the Group or other undesirable consequences. As a result, the Group may not be successful in its efforts to secure suppliers or distribution partners or to develop new cannabis products and produce and distribute these cannabis products. In addition, these activities may require significantly more resources than the Company currently anticipates in order to be successful.
- 48 -
Any new cannabis products that the Group develops or distributes may be subject to time-intensive regulatory approval procedures that might delay any release schedules or lead to adverse market conditions that might affect product profitability. The Group may ultimately fail to effectively bring new product offerings to market for reasons that include, but are not limited to, stringent regulatory approval procedures. Any inability to introduce new product offerings may cause a material adverse effect on the Group's business, results of operations, financial condition and prospects.
Reliance on Third Party Transportation
The Group relies on international third-party transportation services to deliver and receive product-related shipments. In the process of the deliveries, time delays, labor strikes, COVID-19-related issues, product storage issues or other logistical problems may occur and force late delivery or receipt of items or receipt of damaged items. Such delays, receipt of damaged items or other logistical problems may cause a material adverse effect on the Group's business, operations or financial condition. Rising costs associated with courier services used by the Group may also adversely impact the business of the Group and its ability to operate profitably.
In addition, any breach of security of the package during the possession of the third-party transportation service may result in violations of regulations regarding possession of cannabis products and thus may have a material adverse effect on the Group's business, financial condition and operating results.
Investment Business Strategy
As part of the Company's business strategy, it seeks new opportunities in the cannabis industry. In pursuit of such opportunities, the Company may fail to select appropriate investment candidates and negotiate acceptable arrangements. The Company cannot provide assurance that it can complete any investment that it pursues or is pursuing, on favourable terms, or that any investment completed will ultimately benefit the Company. In addition, the Company's capital solutions may not attract a following in the cannabis industry. In the event that the Company chooses to raise debt capital to finance any acquisition or other arrangement, the Company's leverage will be increased. In addition, the introduction of new tax laws or regulations, or accounting rules or policies, or rating agency policies, or changes to, or differing interpretations of, or application of, existing tax laws or regulations or accounting rules or policies or rating agency policies, could make the productivity and services offered by the Company less attractive to investees. Such changes could adversely affect the Company's ability to enter into new investments.
Risks Inherent in Strategic Alliances
The Group may enter into further strategic alliances with third parties that it believes will complement or augment its existing business. The Group's ability to complete strategic alliances is dependent upon, and may be limited by, the availability of suitable candidates and capital. In addition, strategic alliances could present unforeseen integration obstacles or costs, may not enhance the Group's business, and may involve risks that could adversely affect the Group, including significant amounts of management time that may be diverted from investment activities operations to pursue and complete such transactions or maintain such strategic alliances. Future strategic alliances could result in the incurrence of additional debt, costs and contingent liabilities, and there can be no assurance that future strategic alliances will achieve the expected benefits to the Group's business or that the Group will be able to consummate future strategic alliances on satisfactory terms, or at all.
While the Company conducts due diligence with respect to investees, there are risks inherent in any investment. Specifically, there could be unknown or undisclosed risks or liabilities of investees for which the Company is not or will not be sufficiently indemnified. Any such unknown, undisclosed or unmitigated risks or liabilities could materially and adversely affect the Company's financial performance and results of operations. The Company could encounter additional transaction and enforcement related costs or other factors such as the failure to realize all of the benefits from its investments. Any of the foregoing risks and uncertainties could have a material adverse effect on Group's business, financial condition and results of operations.
- 49 -
Risks Associated with Divestment
In certain circumstances, the Company may decide, or be required, to divest any of its direct or indirect interests in certain investees. In particular, if any of the investees violate any applicable laws and regulations, the Company may be required to divest its indirect or direct interest in such investee or risk significant fines, penalties, administrative sanctions, convictions or settlements. There is no assurance that these divestitures will be completed on terms favourable to the Company, or at all. Any opportunities resulting from these divestitures, and the anticipated effects of these divestitures on the Company may never be realized, or may not be realized to the extent the Company anticipates. Any required divestiture or an actual or perceived violation of applicable laws or regulations could have a material adverse effect on the Company, including its reputation and ability to conduct business, its holdings (directly or indirectly) in the investees, the listing of its securities on applicable stock exchanges, its financial position, operating results, profitability or liquidity or the market price of its publicly traded shares. In addition, it is difficult for the Company to estimate the time or resources that may be required for the investigation of any such matters or its final resolution because, in part, the time and resources that may be needed are dependent on the nature and extent of any information requested by the applicable authorities involved, and such time or resources could be substantial.
Evolving Market Competition
There is potential that the Group will face intense competition from other companies or groups of companies, some of which can be expected to have more financial resources, industry, manufacturing and marketing experience than the Group. Because of the early stage of the industry in which the Group operates, as well as evolving legislation and governmental initiatives in a number of jurisdictions, the Group expects to face additional competition from new entrants in the jurisdictions in which it currently operates or is contemplating operations. In particular, the Company expects an increase in market entrants in Germany following the German Local Tender. If the number of users of medical cannabis products in Israel and Europe increases, the demand for products in such areas will increase and the Company expects that competition will become more intense, as current and future competitors begin to offer an increasing number of diversified products and pricing strategies.
As the recreational cannabis market matures in Canada, there is potential that the Company may face intense competition from other domestic and international cannabis brands, cultivators and distributors operating in Canada that are more established or may have access to greater financial resources. In addition, the Company may face intense competition in the Canadian cannabis financing market from financial service providers, including but not limited to banks, credit unions, and alternative lenders, some of which may have longer operating histories and greater financial resources than the Company. Investees may also face intense competition from other companies, some of which can be expected to have longer operating histories and greater financial resources, which may impact investees' ability to service loans issued by the Company. Increased competition by larger and better-financed competitors could materially and adversely affect the business, financial condition and results of operations of the Company.
- 50 -
Industry Consolidation
The cannabis industry is undergoing rapid growth and substantial change, which has resulted in an increase in competitors, consolidation and formation of strategic relationships. It is possible that industry maturation could create larger companies that may have increased geographic scope. Such acquisitions or other consolidating transactions could harm the Group in a number of ways, including the loss of strategic partners (if they are acquired by or enter into relationships with a competitor), customers, or revenue and market share, all of which could harm the Group's operating results. The Group's operating results could also be harmed if the Group was forced to expend greater resources to meet new or additional competitive threats. Additional competition from larger, better-financed competitors with geographic advantages could outcompete the Group by placing downward pressure on retail prices for products and services. This could ultimately cause a material adverse effect on the business, financial condition, results of operations and prospects of the Group.
Reliance on Key Personnel
The Company has relied upon the ability, judgment, discretion and good faith of its executive management team. The Company's future success depends on its continuing ability to attract, develop, motivate and retain highly qualified employees. If the Company were to lose any members of the executive management team or key employees, any inability to find suitable replacements at reasonable costs may have a material adverse effect on the Company's business, financial condition and results of operations.
Reliance on International Advisors and Consultants
The legal and regulatory requirements in the foreign countries in which the Company may invest or operate in with respect to the cultivation and sale of cannabis, banking systems and controls, as well as local business culture and practices are different from those in Canada. The Company's officers and directors must rely, to a great extent, on local legal counsel and consultants in order to keep abreast of material legal, regulatory and governmental developments as they pertain to and affect the Company's business operations, and to assist with governmental relations. The Company must rely, to some extent, on those members of management and the Board who have previous experience working and conducting business in these countries, if any, in order to enhance the Company's understanding of and appreciation for the local business culture and practices. The Company also relies on the advice of local experts and professionals in connection with current and new regulations that develop in respect of the cultivation and sale of cannabis as well as in respect of banking, financing, labour, litigation and tax matters in these jurisdictions. Any developments or changes in such legal, regulatory or governmental requirements or in local business practices are beyond the Company's control. The impact of any such changes may cause a material adverse effect to the Company's business, financial condition, operating results and prospects.
Foreign Market Participation
The Company currently operates or anticipates operating in various international jurisdictions and is subject to inherent risks from the exposure to foreign markets including without limitation currency risk, restrictions on the use of offshore bank accounts for local operating companies; trade restrictions, additional regulatory requirements and restrictions, increased financing costs, litigation risk, high inflation risk, expropriation and nationalization, and political risk. The Company continues to monitor developments and policies in the foreign markets in which it operates or invests and assess the impact thereof to its operations; however, such developments cannot be accurately predicted. The realization of any of these risks may significantly impair the Company's local operations and have a material adverse effect on the Group's business, financial condition and results of operations.
- 51 -
These risks may also limit or disrupt the Group's strategic alliances or investments, restrict the movement of funds, increase the Group's costs, or result in the deprivation of contract rights or the taking of property by nationalization or expropriation without fair compensation, and may have a material adverse effect on the Group's financial position and/or results of operations. In addition, the enforcement by the Group of its legal rights in foreign countries, including rights to exploit properties or utilize permits and licenses and contractual rights may not be recognized by the court systems in such foreign countries or enforced in accordance with the rule of law.
Completion of MYM Transaction
There is no guarantee that the MYM Transaction, as described in "General Development of the Business - Recent Developments - Developments Following the Financial Year Ended December 31, 2020", will be completed in the currently proposed form, if at all, nor is there any guarantee that the Company will be able to continue developing operations in its current jurisdictions or expand into new jurisdictions. Any such activities will require, among other things, various regulatory, court, securityholder, stock exchange and other third-party approvals, licenses and permits and there is no guarantee that all required approvals, licenses and permits will be obtained in a timely fashion or at all.
Future Acquisitions or Dispositions
Material acquisitions, dispositions and other strategic transactions involve a number of risks, including but not limited to the potential disruption of the Group's ongoing business, distraction of management, the Company may become more financial leveraged, the failure to realize anticipated benefits of those transactions fully or at all, or may take longer to realize than expected, and loss or reduction of control over certain Group assets.
Despite the Company's due diligence efforts, the presence of one or more material liabilities of an acquired company that are unknown to the Company at the time of acquisition could have a material adverse effect on the business, results of operations, prospects and financial condition of the Company. A strategic transaction may result in a significant change in the nature of the Company's business, operations and strategy. In addition, the Company may encounter unforeseen obstacles or costs in implementing a strategic transaction or integrating any acquired business into the Company's operations.
In addition, the Company's strategic transaction decisions are based on the economic assessments made by the Company and its external advisors. Such economic assessments involve a series of assumptions regarding factors such as future cannabis prices, production requirements, expected revenue growth, cash flow and financing requirements, future capital expenditures and operating costs. Many of these factors are subject to change and are beyond the control of the Company. If there is any significant negative change in any of these factors, the Company may experience a material adverse effect on its business, financial condition, operating results and prospects.
Management of Growth and Acquisition Integration
The Company may be subject to growth related risks including capacity constraints and pressure on its internal systems and controls. The ability of the Company to manage growth effectively will require it to continue to implement and improve its operational and financial systems and to expand, train and manage its employee base. If the Company is unable to deal with this growth, any negative impact may have a material adverse effect on the Company's business, financial condition, results of operation and prospects.
- 52 -
In addition, the realization of the benefits of acquisitions made by the Company, including the acquisition of Trichome, depend in part on successfully consolidating functions and integrating and leveraging operations, procedures and personnel in a timely and efficient manner as well as the Company's ability to share knowledge and realize revenues, synergies and other growth opportunities from combining the acquired businesses and operations with those of the Company. The integration of acquired businesses may depend on a number of factors, including without limitation: (i) the input of substantial management effort, time and resources; (ii) the successful incorporation of key personnel from acquired companies for post-acquisition periods; and (iii) the execution of effective non-competition agreements with certain employees or ex-employees of the acquired companies. Any failure in successfully integrating acquired businesses may result in a material adverse effect on the Company's business, financial condition, operating results and prospects.
Foreign Expansion Efforts and Operations
The Company's expansion into foreign jurisdictions is subject to additional business risks, including new or unexpected risks or could significantly increase the Company's exposure to one or more existing risk factors, including economic instability, changes in laws and regulations, and the effects of competition, as well as operational, regulatory, compliance and reputational and foreign exchange rate risk. In addition, future international expansion could require the Group to incur a number of up-front expenses, including those associated with obtaining regulatory approvals, as well as additional ongoing expenses, including those associated with infrastructure, staff and regulatory compliance. The failure of the Company's operating infrastructure to support such expansions could result in operational failures and regulatory fines or sanctions. Additionally, there is no guarantee that the Company will be able to realize any of the anticipated benefits of any transactions related to the Company's expansion strategy.
U.S. Operations
The Company and, to its knowledge, its investees, do not currently engage in any U.S. cannabis-related activities as defined in CSA Staff Notice 51-352. To date, the Company has caused its investees to only conduct business and invest in entities in federally-legal jurisdictions by including appropriate representations, warranties and covenants in its agreements with investees. However, an investee may breach such obligations. Any such violation of such obligation would result in a breach of the applicable agreement and, accordingly, may have a material adverse effect on the business, operations and financial condition of Company.
Conditions in Israel
The Group is vulnerable to the political, economic, legal, regulatory, and military conditions affecting Israel and the Middle East. Armed conflicts between Israel and its neighbouring countries and territories occur periodically in the region and may adversely affect the Group's business, results of operations and financial condition. In addition, the Group may be adversely affected by other events or factors affecting Israel such as the interruption or curtailment of trade between Israel and its trading partners, or any restrictions or pressure on the Group's partners or customers or others to prevent or discourage them from doing business activities with Israel or Israeli businesses, a significant downturn in the economic or financial condition of Israel, a significant downgrading of Israel's internal credit rating, labour disputes and political instability, including riots, uprisings and government failures. Restrictive laws or policies directed towards Israel or Israeli businesses could have a material adverse effect on the Group's business, results of operations, financial condition and prospects.
- 53 -
Furthermore, under Israeli law, citizens and permanent residents of Israel are obligated to perform military reserve duty for extended periods of time and are subject to being called to active duty at any time under emergency circumstances. In response to increased hostilities, there have been periods of significant call-ups of military reservists. It is possible that there will be additional call-ups in the future, which may include officers and key personnel of the Group, which could disrupt business operations for a significant period of time.
Political Risk
As mentioned in "Foreign Market Participation" above, political risk is an additional risk that the Group may be exposed to when operating in a foreign market. Examples of political risk include without limitation social unrest, threats or occurrences of war, organized crime, political instability, changes of government and changes in taxation policies.
Some foreign markets are prone to higher levels of political risk. Emerging markets tend to have particularly sensitive political and social environments where governments may be capable of wide-sweeping executive actions that may materially impact the local cannabis market or other markets relevant to the Company. Such actions can include without limitation enactment of price controls, trade restrictions, taxes, land and property regulations, and environmental restrictions.
While the Company actively analyzes risks and developments in foreign markets that it currently or will participate in, there is no assurance that unpredicted impacts will not occur. Depending on the magnitude of such unpredicted impacts, there may be a material adverse effect on the Company's business, financial condition, operating results and prospects.
Inflation in Emerging Markets
In the past, high levels of inflation have adversely affected emerging economies and financial markets, and the ability of government to create conditions that stimulate or maintain economic growth. Moreover, governmental measures to curb inflation and speculation about possible future governmental measures have contributed to the negative economic impact of inflation and have created general economic uncertainty.
The emerging markets in which the Group operates or may operate may experience high levels of inflation in the future. Inflationary pressures may weaken investor confidence in such countries and lead to further government intervention in the economy. If countries in which the Group operates experience high levels of inflation in the future and/or price controls are imposed, the Company may not be able to adjust the rates the Group charges its customers to fully offset the impact of inflation on the Company's cost structures, which could cause a material adverse effect on the Company's business, financial condition, results of operations and prospects.
Acquisition or Use of Properties in Foreign Jurisdictions
Non-resident individuals and non-domiciled foreign legal entities may be subject to restrictions on the acquisition or lease of properties in certain emerging markets. Limitations also apply to legal entities domiciled in such countries that are controlled by foreign investors, such as the entities through which the Group operates in certain countries. Accordingly, the Company's current and future operations may be impaired as a result of such restrictions on the acquisition or use of property, and the Group's ownership or access rights in respect of any property it owns or leases in such jurisdictions may be subject to legal challenges, all of which could result in a material adverse effect on the Company's business, financial condition, results of operations and prospects.
- 54 -
Risks Inherent in the Agricultural Business
The Company's business, specifically as it pertains to Adjupharm, TJAC and the Company's relationship with Focus, involves the growing of cannabis products, which are agricultural products. As such, the business is subject to the risks inherent in the agricultural business, such as pests, plant diseases and similar agricultural risks. Although TJAC, Focus and Adjupharm and their respective third-party cultivators carefully monitor the growing conditions with trained personnel and applicable equipment, there can be no assurance that natural elements will not have a material adverse effect on the production of its products and results of operations of TJAC, Focus or Adjupharm. Any decline in production by TJAC, Focus or Adjupharm could have a material adverse effect on the Company's business, operating results or financial condition.
Illegal Market Competition
As a participant of the cannabis market in international jurisdictions with varying regulations, the Company may be subject to competition from entities that conduct illegal cannabis business operations. Such entities may resort to competitive measures such as producing products with prohibited concentrations of THC and CBD or producing imitations of IMC-branded products without the authorization or endorsement of the Company. If demand for these illegal products increases and local governments fail to regulate markets accordingly, the Company may experience a material adverse effect on its business, operating results and prospects.
Restrictions on Sales and Marketing
The industry is in its early development stage and restrictions on sales and marketing activities imposed by cannabis regulatory authorities, various medical associations, other governmental or quasi-governmental bodies or voluntary industry associations in the jurisdictions in which the Group operates may adversely affect the Group's ability to conduct sales and marketing activities and could have a material adverse effect on the Company's business, operating results, financial condition and prospects.
The Group's success depends on its ability to attract and retain customers. The way cannabis products are packaged, labelled, and displayed is strictly regulated in the jurisdictions in which the Group operates. For example, advertising related to consumption of cannabis is strictly prohibited in Israel. Such prohibitions may affect the Company's ability to establish brand presence, acquire new customers, retain existing customers and maintain a loyal customer base. This may ultimately have a material adverse effect on the Group's business, financial conditions and operations.
Publicity and Consumer Perception
The Company believes the medical cannabis industry is highly dependent upon consumer perception regarding the safety, efficiency and quality of the medical cannabis products produced. Consumer perception of the Group's products can be significantly influenced by scientific research or findings, regulatory investigations, litigation, media attention and other publicity regarding the consumption of medical cannabis products.
There can be no assurance that future scientific research, findings, regulatory proceedings, litigation, media attention or other research findings or publicity will be favorable to the medical cannabis market or any particular product, or consistent with earlier publicity.
Future research reports, findings, regulatory proceedings, litigation, media attention or other publicity that are perceived as less favorable than, or that question, earlier research reports, findings or publicity could have a material adverse effect on the demand for products bearing the IMC brand and the business, results of operations, financial condition, prospects and the Company's cash flows. The Company's dependence upon consumer perceptions means that adverse scientific research reports, findings, regulatory proceedings, litigation, media attention or other publicity, whether or not accurate or with merit, could have a material adverse effect on the Company, the demand for products bearing the IMC brand, and the business, results of operations, prospects, financial condition and cash flows of the Company.
- 55 -
Further, adverse publicity reports or other media attention regarding the safety, efficacy and quality of medical cannabis products in general, or the Group's products specifically, or associating the consumption of medical cannabis products with illness or other negative effects or events, could have a material adverse effect. Such adverse publicity reports or other media attention could arise even if the adverse effects associated with such products resulted from consumers' failure to consume such products appropriately or as directed.
Perceived Effects of Products
If the products the Group sells are not perceived to have the effects intended by the end user, its business may suffer. There is little long-term data with respect to efficacy, unknown side effects and/or interaction with individual human biochemistry of various cannabis products. As a result, the Group's products could have certain side effects if not taken as directed or if taken by an end user that has certain known or unknown medical conditions.
Reputational Risk to Third Parties
The parties outside of the cannabis industry with which the Group does business may perceive that they are exposed to reputational risk as a result of the Group's cannabis business activities. Failure to establish or maintain business relationships could have a material adverse effect on the Group's business, financial condition, results of operations and prospects.
Information Technology
The Group's operations will depend, in part, on how well it and its supply and distribution partners protect networks, equipment, information technology systems ("IT systems") and software against damage from a number of threats, including, but not limited to, cable cuts, damage to physical plants, natural disasters, intentional damage and destruction, fire, power loss, hacking, computer viruses, vandalism and theft. The Group's operations also will depend on the timely maintenance, upgrade and replacement of networks, equipment, IT systems and software, as well as pre-emptive expenses to mitigate the risks of failures. Any of these and other events could result in information system failures, delays and/or increase in capital expenses. The failure of IT systems or a component of IT systems could, depending on the nature of any such failure, adversely impact the Group's financial condition, operating results and reputation.
Cybersecurity
The Group's information systems and its third-party service providers and vendors are vulnerable to increasing threat of continually evolving cybersecurity risks, resulting in data breaches and data losses. These risks arising from events including without limitation malware, computer viruses, employee error, extortion, malfeasance, system errors, hacking. In order to minimize the risk of these events from occurring, the Group is performing timely maintenance, upgrade and replacement of networks, equipment, IT systems and software and other protective measures. However, any failure or delay in maintaining, upgrading or replacing such systems and software could materially increase the risk of cybersecurity incident and data breach or data loss, and the Group may experience operational delays, information system failures, and/or increases in capital expenses. Ultimately, the Company's business, financial condition, operating results and reputation may be impacted adversely by such occurrences.
- 56 -
The Group has not experienced any material losses to date relating to cybersecurity-attacks or other information security breaches, but there can be no assurance that the Group will not incur such losses in the future. The Group's risk and exposure to these matters cannot be fully mitigated because of, among other things, the evolving nature of these threats. As a result, cyber security and the continued development and enhancement of controls, processes and practices designed to protect systems, computers, software, data and networks from attack, damage or unauthorized access is a priority. As cyber threats continue to evolve, the Group may be required to expend additional resources to modify or enhance protective measures or to investigate and remediate any security vulnerabilities.
Privacy
The Group collects and stores certain personal information about its patients and customers, and is responsible for protecting that information from privacy breaches. A privacy breach may occur through certain threats, including, without limitation, procedural or process failure, information technology malfunction, or deliberate unauthorized intrusions, computer viruses, and cyber-attacks. Theft of data for competitive purposes is an ongoing risk whether perpetrated via employee collusion or negligence or through deliberate cyber-attack. Any such theft or privacy breach would have a material adverse effect on the Group's business, financial condition and results of operations.
In addition, there are a number of Israeli, German, European, and Canadian federal and provincial laws protecting the privacy and confidentiality of certain patient health information, including patient records, and employee information, and restricting the collection, use and disclosure of that protected information. In Canada, the privacy rules under PIPEDA and provincial statutes regulating the collection, use and disclosure of personal information, protect medical records and other personal health information by limiting their use and disclosure of health information to the minimum level reasonably necessary to accomplish the intended purpose. If the Group was found to be in violation of the privacy or security rules under PIPEDA or other applicable privacy laws protecting the privacy and confidentiality of patient health information in the jurisdictions in which it operates, the Group could be subject to sanctions and civil or criminal penalties, which could increase its liabilities, harm its reputation and have a material adverse effect on the business, financial condition and operating results of the Group. In addition, the EU's GDPR governs the collection and use of personal data in the European Union. The GDPR, which is wide-ranging in scope, will impose several requirements relating to the consent of the individuals to whom the personal data relates, the information provided to the individuals, the security and confidentiality of the personal data, data breach notification and the use of third-party processors in connection with the processing of the personal data. The GDPR also imposes strict rules on the transfer of personal data out of the EU to the U.S., enhances enforcement authority and imposes large penalties for noncompliance, including the potential for fines of up to EUR 20 million or four percent of the annual global revenues of the infringer, whichever is greater. In addition, certain breaches of the GDPR may result in regulatory investigations, reputational damage and civil lawsuits including class action lawsuits. In the State of Israel, privacy rights and obligations are mainly regulated under the Protection of Privacy Law, 5741-1981 (the "Israeli Privacy Law") and the regulations promulgated thereunder (mainly the Protection of Privacy (Data Security) Regulations, 5777-2017 and the Protection of Privacy (Transfer of Data Abroad) Regulations, 5761-2001) (the "Israeli Privacy Regulations"). Under the Israeli Privacy Law, 'information' and 'sensitive information' includes information such as those related to a person's health, personality, intimate affairs, financial condition, faith and opinions. The Israeli Privacy Law impose obligations related to database registration, notice, disclosure and use restrictions on an 'owner' of a database, and the Israeli Privacy Regulations set forth the security measurements to be implemented and the rules related to the transfer of personal information. Violation of the Israeli Privacy Law could lead to a criminal investigation or an administrative enforcement procedure on behalf of the Israeli Privacy Protection Authority, as well as an administrative fine imposed pursuant to the Administrative Offenses Law, 5746-1985. In addition, legal remedies such as statutory compensation of up to NIS 50,000 are available to successful claimants of privacy violations.
- 57 -
Additional jurisdictions in which the Group operates or in which it may enter in the future, also have data privacy and security laws and regulations that govern the collection, use, disclosure, transfer, storage, disposal, and protection of sensitive personal information. The interpretation and enforcement of such laws and regulations are uncertain, are subject to change and may require the Group to incur substantial costs to monitor and implement compliance with any additional requirements. Failure to comply with data protection laws and regulations could result in government enforcement actions, litigation and/or adverse publicity and could negatively affect the Group's operating results, business and prospects.
Wholesale Price Volatility
The cannabis industry is a margin-based business in which gross profits depend on the excess of sales prices over costs. Consequently, profitability is sensitive to fluctuations in wholesale and retail prices caused by changes in supply (which itself depends on other factors such as weather, fuel, equipment and labour costs, shipping costs, economic situation, government regulations and demand), taxes, government programs and policies for the cannabis industry (including price controls and wholesale price restrictions that may be imposed by government agencies responsible for the sale of cannabis), and other market conditions, all of which are factors beyond the control of the Group. The Group's operating incomes may be significantly and adversely affected by a decline in the price of cannabis products and will be sensitive to changes in the price of cannabis products and the overall condition of the cannabis industry, as the Group's profitability is directly related to the price of cannabis products. The price of cannabis products is affected by numerous factors beyond the Group's control. Any price decline may have a material adverse effect on the Group's business, financial condition and results of operations.
Risks Inherent in Investments
The Company is not directly involved in the ownership or operation of and may have limited contractual rights relating to the operations of its current and future investee entities. An investee generally has the power to determine the manner in which its business is developed, expanded and operated, and the Company's interest in an investee is subject to the risks applicable to the business carried on by the investee, and the Company may fail to realize all of the potential benefits from its investments. The interests of the Company and its investees may not always be aligned. As a result, any cash flows of the Company from investees will be dependent upon the activities of the investees, which creates the risk that at any time those investees may: (i) have business interests or targets that are inconsistent with those of the Company; (ii) take action contrary to the Company's policies or objectives; (iii) be unable or unwilling to fulfill their obligations under their agreements with the Company; (iv) experience financial, operational or other difficulties, including insolvency, which could limit or suspend an investee's ability to perform its obligations under agreements with the Company or (v) fail to comply with applicable laws or best practices.
Fraudulent or Illegal Activity
The Group's employees, independent contractors and consultants may expose the Group to additional risk if they engage in fraudulent or other illegal activity prohibited by relevant laws. Although the Group has set preventative measures in place to minimize such fraud or illegal activities from occurring, there is no guarantee that the measures will be effective. If the measures fail and fraud or illegal activities take place, the Group may be subject to lawsuits for failure to comply with regulations and be ordered to pay such penalties as prescribed by the court if found to be in violation. Thus, the occurrence of fraud or illegal activities may cause a material adverse effect on the Group's business, reputation, financial condition and results of operations.
- 58 -
Corruption and Anti-Bribery Law Violations
The Company's business is subject to Canadian laws, which generally prohibit companies and employees from engaging in bribery or other prohibited payments to foreign officials for the purpose of obtaining or retaining business. In addition, the Group is subject to the anti-bribery laws of any other countries in which it conducts business now or in the future. The Group's employees or other agents may, without its knowledge and despite its efforts, engage in prohibited conduct under the Group's policies and procedures and anti-bribery laws for which the Group may be held responsible. The Company's policies mandate compliance with these anti-corruption and anti-bribery laws. However, there can be no assurance that the Company's internal control policies and procedures will always protect it from recklessness, fraudulent behaviour, dishonesty or other inappropriate acts committed by its affiliates, employees, contractors or agents. If the Group's employees or other agents are found to have engaged in such practices, the Group could suffer severe penalties and other consequences that may have a material adverse effect on its business, reputation, financial condition and results of operations.
Intellectual Property
The Group uses intellectual property protections such as trademarks, trade secrets and contractual confidentiality obligations in order to protect its products, brands and technologies. The administrative task of maintaining such protections across multiple jurisdictions can result in high costs to the Group. The Group would also be required to pay for any costs attributed to the enforcement of intellectual property protections. In addition, in any infringement proceeding, some or all of the Group's intellectual property rights or other proprietary know-how, may be found invalid, unenforceable, anti-competitive or not infringed. An adverse result in any litigation or defense proceedings could create the risk of invalidation or narrow interpretation of the Group's affected intellectual property rights. Such results could cause a material adverse effect on the Group's business, financial condition, results of operations and prospects.
Furthermore, the possession of intellectual property protections does not completely eliminate the risk of litigation. Even with such protections properly registered, the Group is still vulnerable to infringement claims and would be liable for the costs of defending such claims. If the claims succeed, the Group would be liable for the costs of the resulting court orders and may need to negotiate licensing of the intellectual property rights from third-party owners.
In light of the above, the Group makes no assurances regarding any potential costs paid towards intellectual property fees or terms of licenses negotiated.
In addition, despite any intellectual property protections in place, unauthorized parties may attempt to replicate or otherwise obtain and use the Group's trademarks, know-how, trade secrets, products or technology. Identifying unauthorized use of intellectual property rights is difficult as the Group may be unable to effectively monitor and evaluate the products being distributed by its competitors, including parties such as illegal distributers, and the processes used to produce such products. The Group makes no assurance that it will successfully identify unauthorized replication, acquisition or use of the Group's trademarks, know-how, trade secrets, products, or technology before the effects of such actions cause a material adverse effect on the Group's business, financial condition, results of operation and prospects.
- 59 -
CSE and NASDAQ Continued Listing Requirements
The Common Shares and Warrants began trading on the CSE on November 5, 2019 and November 19, 2019, respectively. The Common Shares began trading on NASDAQ on March 1, 2021.
The Company is subject to the rules and regulations of NASDAQ and the CSE. Further, in order to maintain compliance with all continued listing requirements, the Company pays legal, accounting and compliance fees to advisors and regulatory organizations and will have to continue to pay additional fees if its Common Shares remain listed on NASDAQ. Any changes to rules, regulations policies or guidelines issued by regulatory authorities may impact any such fees paid and increase the risk of non-compliance. There is no assurance that the Company will be able to comply with applicable NASDAQ continued listing standards within any projected timeframes, or at all, and maintain listing status on either NASDAQ or CSE.
Any failure to comply with applicable continued listing requirements and regulations may result in the delisting of the Company's Common Shares and/or Warrants from the CSE and/or the Company's Common Shares from NASDAQ. Such events may have material adverse effects on the Company's business and financial condition.
Significant Sales of Listed Securities
Sales of a substantial number of Common Shares or other equity-related securities in the public markets by the Company or its shareholders could depress the market price of the Company's securities and impair the Company's ability to raise capital through the sale of additional equity securities. The Company cannot predict the effect that future sales of Common Shares or other equity-related securities would have on the market price of the Common Shares or Listed Warrants. The price of the Common Shares or Listed Warrants could be affected by possible sales of the Common Shares or Listed Warrants by hedging or arbitrage trading activity.
Dilution
The Company may issue additional securities in the future, which may dilute a shareholder's holdings, or a holder of a convertible security's underlying relative interest, in the Company. The Company's articles permit the issuance of an unlimited number of Common Shares, and shareholders will have no pre-emptive rights in connection with any such further issuance. The directors of the Company have discretion to determine the price and the terms of further issuances, subject to applicable stock exchange policies. Moreover, additional Common Shares will be issued by the Company on the full exercise of Options, Broker Options, RSUs and Warrants, issued or to be issued by the Company in the future, and the exercise of any resulting convertible securities of such as applicable.
As of the date of this Annual Information Form, the issuances of Options are limited by the Option Cap. Subject to shareholder approval, the Option Cap may be increased to a higher percentage of Common Shares issued and outstanding. As a result, additional dilution may occur if more options are issued under an increased Option Cap.
- 60 -
Additionally, on March 31, 2021, the Company filed the Final Shelf Prospectus which allows the Company to offer for sale up to USD 250,000,000 in Qualified Securities of the Company for an effective period of 25 months. Any issuances of Qualified Securities under a supplement to the Final Shelf Prospectus may dilute a shareholder's holdings or a holder of a convertible security's underlying relative interest, in the Company.
Holding Company Status
IMCC is a holding company. Substantially all of the Company's operating assets are the capital stock of its subsidiaries and arrangements with investees. Substantially all of the Company's business is conducted through subsidiaries or investees which are separate legal entities. Consequently, the Company's cash flows and ability to pursue future business and expansion opportunities are dependent on the earnings of its subsidiaries and investees and the distribution of those earnings to the Company. The ability of these entities to pay dividends and other distributions will depend on their operating results and will be subject to applicable laws and regulations which require that solvency and capital standards be maintained by such companies and contractual restrictions contained in the instruments governing their debt. In the event of a bankruptcy, liquidation or reorganization of any of the Company's subsidiaries, holders of indebtedness and trade creditors will generally be entitled to payment of their claims from the assets of those subsidiaries before any assets are made available for distribution to the Company.
Dividends
The Company has not paid any dividends on the outstanding Common Shares, and the Company maintains no current intention to declare dividends on the Common Shares in the foreseeable future. Any decision to pay dividends on the Common Shares in the future will be at the discretion of the Board and will depend on, among other things, the Company's results of operations, current and anticipated cash requirements and surplus, financial condition, any future contractual restrictions and financing agreement covenants, solvency tests imposed by corporate law and other factors that the Board may deem relevant. As a result, investors may not receive any return on an investment in the Common Shares unless they are able to sell their Common Shares for a price greater than that which such investors paid for them.
Securities Price Volatility
The market price of the Common Shares and Warrants may fluctuate to a wide degree as a result of a number of factors, including without limitation market conditions, financial analyst predictions, changes in law, press releases and public filings of the Company and competitor activity. In particular, the dual-listing of the Common Shares on the CSE and the NASDAQ may result in higher volatility as a result of the exposure to both U.S. and Canadian financial market conditions. Overall, such factors, whether related or unrelated to operational performance of the Company, may cause a temporary or non-temporary negative pressure on prices of the Company's securities or assets. If the negative pressure on prices arising from these factors persist, impairment losses may be recorded and the Company could experience a material adverse effect on its operations, financial condition and operating results.
Internal Controls
Effective internal controls are required for the Company to provide reasonable assurance that its financial results and other financial information are accurate and reliable. Any failure to design, develop or maintain effective controls, or difficulties encountered in implementing, improving or remediation lapses in internal controls may affect the Company's ability to prevent fraud, detect material misstatements, and fulfill our reporting obligations. As a result, investors may lose confidence in the Company's ability to report timely, accurate and reliable financial and other information, which may expose the Company to certain legal or regulatory actions, thus negatively impacting its business and financial condition, including the liquidity and/or market value of its securities.
- 61 -
Liquidity of Securities
Despite the listing of the Common Shares and Warrants on public exchanges, there is no guarantee to security holders that the securities will be sufficiently liquid to any degree without a substantial decrease in price, particularly if selling significant quantities within a short time frame. Accordingly, there is a possibility that a lack of liquidity may cause difficulty for security holders to re-sell securities at desired prices.
Credit Risk
The Group may be owed current or long-term debts such as accounts receivables over the course of its operations. As a result, the Group may be exposed to the risk of debtor defaults on payments as they come due. This credit risk can be mitigated by the Group through a number of options including, without limitation, taking collateral, obtaining guarantees, and negotiating credit agreements. The Company makes no guarantee on the level of credit risk that it will hold at any given time but intends to minimize this risk as determined by the Board.
The Company is exposed to counterparty risks including, but not limited to: (i) through the investees which may experience financial, operational or other difficulties, including insolvency, which could limit or suspend those investees' ability to perform their obligations under agreements with the Company or result in the impairment or inability to recover the Company's equity investment in an investee; (ii) through financial institutions that may hold the Company's cash and cash equivalents; (iii) through companies that have payables to the Company; (iv) through Company's insurance providers; and (v) through the Company's lenders, if any.
Liquidity Risk
The Group is subject to the inherent risk that it will not be able to pay its financial obligations as they become due. In light of its recent negative cash flows, the Company intends to monitor liquidity risk carefully and plan its liquid holdings strategically to avoid any payment defaults.
Market Risk
Market risk is the risk that the fair value or future cash flows of a financial instrument will fluctuate because of changes in market prices. Market risk includes exchange rate risk and interest rate risk.
Exchange rate risk is the risk of loss arising from changes to foreign exchange rates. As the Group is a party to certain international contracts that require the Company to make or receive payments in foreign currencies, there is a risk that losses will be incurred if there is an adverse shift in exchange rates.
Interest rate risk pertains to the risk of loss arising from changes in prevailing interest rates. Any increases in prevailing interest rates may increase interest expenses paid by the Group on any long-term debt.
- 62 -
Global Economy Risk
An economic downturn of global capital markets has been shown to make the raising of capital by equity or debt financing more difficult. The Company will be dependent upon the capital markets to raise additional financing in the future, while it continues to develop its operations. As such, the Company is subject to liquidity risks in meeting its development and future operating cost requirements in instances where cash positions are unable to be maintained or appropriate financing is unavailable. These factors may impact the Company's ability to raise equity or obtain loans and other credit facilities in the future and on terms favorable to the Company and its management. If uncertain market conditions persist, the Company's ability to raise capital could be jeopardized, which could have an adverse impact on the Company's operations and the trading price of the Company's securities.
Further, global credit and financial markets have displayed arguably increased volatility in response to global events. For instance, since November 30, 2019, the COVID-19 pandemic resulted in governments worldwide enacting emergency measures to combat the spread of COVID-19. These measures, which include the implementation of travel bans, self-imposed quarantine periods and social distancing, have caused material disruption to businesses globally resulting in an economic slowdown. Global equity markets have experienced significant volatility and weakness. Governments and central banks have reacted with significant monetary and fiscal interventions designed to stabilize economic conditions. The duration and impact of the COVID-19 pandemic are unknown at this time, as is the efficacy of government and central bank interventions. It is not possible to reliably estimate the length and severity of these developments and the impact on the Group's business, financial condition, results of operations and prospects.
Future crises may be precipitated by any number of causes, including natural disasters, public health crises, geopolitical instability, natural disasters, changes to energy prices or sovereign defaults. These factors may impact the ability of the Company to obtain equity or debt financing in the future and, if obtained, on terms favorable to the Company. Increased levels of volatility and market turmoil can adversely impact the Group's operations and the value, and the price of the Common Shares and/or Warrants could be adversely affected.
In addition, there is a risk that one or more of the Group's current service providers may themselves be adversely impacted by difficult economic circumstances, which could have a material adverse effect on the Group's business, financial condition, results of operations and prospects.
Sufficiency of Insurance
The Group maintains various types of insurance which may include product liability insurance (see "Potential Product Liability" below), errors and omission insurance, directors and officers insurance, trustees' insurance, property coverage and general commercial insurance. There is no assurance that claims will not exceed the limits of available coverage, that any insurer will remain solvent or willing to continue providing insurance coverage will sufficient limits or at a reasonable cost; or, that any insurer will not dispute coverage of certain claims due to ambiguities in the policies. A judgment against any member of the Group in excess of available coverage could have a material adverse effect on the Group in terms of damages awarded and negatively impact the reputation of the Group.
Uninsured or Uninsurable Risks
The insurance purchased by the Group cannot cover all risks that the Group is exposed to. Additionally, some insurance policies are outside of budget limitations and are therefore elected to be excluded. There is no guarantee that any insurance coverage maintained by any member(s) of the Group will sufficiently cover any or all liabilities incurred by that Group member. Any uninsured amounts of liabilities incurred by member(s) of the Group may be paid directly by such members. Accordingly, such direct payments may have a material adverse effect on the Group's business, results of operations, and financial condition.
- 63 -
Potential Product Liability
The Company derives a significant portion of its revenues from Focus and Adjupharm and it expects to receive a significant portion of its revenue from TJAC commencing on March 18, 2021. Focus, Adjupharm and TJAC are producers and/or distributors of products designed to be ingested or inhaled by humans. Such products face an inherent risk of exposure to product liability claims, regulatory action and litigation if such products are alleged to have caused significant loss or injury. In addition, the manufacture and sale of such products involve the risk of injury or loss to consumers due to tampering by unauthorized third parties, product contamination, unauthorized use by consumers or other third parties. Previously unknown adverse reactions resulting from human consumption of cannabis products alone or in combination with other medications or substances could occur.
The Group may be subject to various product liability claims, including, among others, that products manufactured, distributed by the Group or bearing one of the Group's brands caused injury, illness or loss, include inadequate instructions for use or include inadequate warnings concerning possible side effects or interactions with other substances. A product liability claim or regulatory action against the Group could result in increased costs, could adversely affect the Group's reputation with its clients and consumers generally, and could have a material adverse effect on the Group's results of operations and financial condition.
There can be no assurances that the Group will be able to obtain or maintain product liability insurance on acceptable terms or with adequate coverage against potential liabilities. Such insurance is expensive and may not be available in the future on acceptable terms, or at all. The inability to obtain sufficient insurance coverage on reasonable terms or to otherwise protect against potential product liability claims could prevent or inhibit the commercialization of the Group's products.
Current and Potential General Litigation
Certain members and/or representatives of the Group are parties to certain legal proceedings or investigations, including the Construction Allegations, the MOH Allegations and certain legal proceedings as described in "Legal Proceedings and Regulatory Actions - Legal Proceedings" below. Should such Group members and/or representatives fail to receive favorable decisions at the conclusion of these legal proceedings or incur significant costs in litigation thereof, the Group's business, financial condition or operating results may be subject to a material adverse effect.
Members and/or representatives of the Group are or may become parties to litigation from time to time in the ordinary course of business that could adversely affect its business. Should any litigation in which the Group members and/or representatives become involved be determined against such Group members and/or representatives, such a decision could adversely affect the Group's ability to continue operating and the market price for the Common Shares and/or Warrants. Even if such Group members and/or representatives are involved in litigation and win, the litigation process can consume significant resources of the Group.
- 64 -
Quality Control Systems
The quality and safety of IMC-branded products are critical to the success of the Group's business and operations. As such, it is imperative that the Group's (and its service providers') quality control systems operate effectively and successfully. Quality control systems can be negatively impacted by the design of the quality control systems, the quality training program, and adherence by employees to quality control guidelines. Although the Group strives to ensure that it and all of its service providers have implemented and adhere to high calibre quality control systems, the Group could experience a significant failure or deterioration of such quality control systems. A failure of the Group's quality control systems could result in significant costs incurred in replacing, destroying or repurposing defective inventory, providing replacement products to its customers or recalling such products. The Group may be unable to meet customer demand and may lose customers who have to purchase alternative brands or products. In addition, consumers may lose confidence in IMC-branded products whether affected or not and the IMC brand may be materially damaged. Any loss of sales volume from a contamination event may affect the Group's ability to fulfill its contractual obligations. During this time, the Group's competitors may benefit from an increased market share that could be difficult and costly to regain.
Potential Product Recalls
Manufacturers and distributors of products are sometimes subject to the recall or return of their products for a variety of reasons, including product defects, such as contamination, unintended harmful side effects or interactions with other substances, packaging safety and inadequate or inaccurate labeling disclosure. If products bearing the IMC brand are recalled due to an alleged product defect or for any other reason, the Group could be required to incur the unexpected expense of the recall and any legal proceedings that might arise in connection with the recall.
The Group may lose a significant amount of sales and may not be able to replace those sales at an acceptable margin or at all. In addition, a product recall may require significant management attention.
Although the Group has detailed procedures in place for testing finished products , there can be no assurance that any quality, potency or contamination problems will be detected in time to avoid unforeseen product recalls, regulatory action or lawsuits. Additionally, if one of the Company's significant brands were subject to recall, the image of that brand and the Group could be harmed. A recall for any one of the foregoing reasons could lead to decreased demand for the Group's products and could have a material adverse effect on the results of operations and financial condition of the Group. Additionally, product recalls may lead to increased scrutiny of the Group's operations by the MOH or other regulatory agencies, requiring further management attention and potential legal fees and other expenses.
Difficulty in Forecasts
The Company's sales forecasts are largely dependent on the Company's own market research. There is no assurance pertaining to the accuracy of the Company's predictions regarding the cannabis industry. Any assumptions made in producing forecasts may be inaccurate as a result of external factors that are unpredictable to the Group. Such inaccuracies could have a material adverse effect on the Group's business, financial condition and results of operations.
Catastrophic Events, Natural Disasters, Severe Weather and Disease
The Group's business may be negatively impacted by a number of events that are beyond its control, including cyber-attacks, energy blackouts, pandemics, terrorist attacks, acts of war, earthquakes, hurricanes, tornados, fires, floods, ice storms or other catastrophic events. Further, the Group relies on certain suppliers and distribution partners whose businesses may be impacted by the occurrence of any of the foregoing events. Catastrophic events can evolve rapidly and their impacts can be difficult to predict. There can be no assurance that the occurrence of a catastrophic event or the associated consequences will not disrupt the Group's operations, ability to carry on business or supply and distribution chains. A catastrophic event, including an outbreak of infectious disease, a pandemic or a similar health threat, such as the COVID-19 pandemic, or fear of any of the foregoing, could adversely impact the Group and its ability to maintain normal operations. In addition, liquidity and volatility, credit availability, market and financial conditions and cannabis cultivation, supply and distribution conditions, among other critical factors to the Group's business, could change at any time as a result. These events and any associated consequences may cause a material adverse effect on the business, financial condition and results of operations of the Group.
- 65 -
Anti-Money Laundering Laws and Regulation Risks
The Group is subject to a variety of laws and regulations domestically and internationally that involve money laundering, financial recordkeeping and proceeds of crime, including the Proceeds of Crime (Money Laundering) and Terrorist Financing Act (Canada), as amended and the rules and regulations thereunder, the Criminal Code (Canada) and any related or similar rules, regulations or guidelines, issued, administered or enforced by governmental authorities internationally. In the event that any of the Group's investments, or any proceeds thereof, any dividends or distributions therefrom, or any profits or revenues accruing from such investments were found to be contrary to money laundering legislation, such transactions may be viewed as proceeds of crime under one or more of the statutes noted above or any other applicable legislation. This could restrict or otherwise jeopardize the ability of the Company to declare or pay dividends, effect other distributions or subsequently repatriate such funds back to Canada. Furthermore, while the Company has no current intention to declare or pay dividends in the foreseeable future, in the event that Company determines to declare or pay dividends but a determination was made that the investments in Company's investees could reasonably be shown to constitute proceeds of crime, Company may subsequently decide or be required to suspend declaring or paying dividends without advance notice and for an indefinite period of time.
Security over Underlying Assets
There is no guarantee that the Group will be able to effectively enforce any guarantees, indemnities or other security interests it may have. Should a bankruptcy or other similar event occur that precludes an investee from performing its obligations under an agreement with any member of the Group, the Group would have to enforce its security interest. In the event that the investee has insufficient assets to pay its liabilities, it is possible that other liabilities will be satisfied prior to the liabilities owed to the Group. In addition, bankruptcy or other similar proceedings are often a complex, lengthy and expensive process, the outcome of which may be uncertain and could result in a material adverse effect on the Company.
In Canada, there is a gap in the regulatory scheme as it applies to the ability of lenders to secure collateral, including regulated assets and regulatory licences themselves. In the event of a default, it is currently unclear how or if a lender would be able to realize on its security because it is unclear whether security can be taken in the relevant cannabis licences themselves, whether cannabis licences may be transferred in such circumstances, and whether a lender could take possession of regulated collateral. Canadian cannabis regulations are silent on these topics, and accordingly there can be no assurance that a lender in the cannabis industry will be in a position to enforce security in regulated assets or regulatory licences. The Company continues to monitor market practice and legal developments in this area. The Company seeks to mitigate risks on its loan portfolio through a variety of methods other than security, and with respect to the taking and enforcement of security, intends to work closely with legal counsel and regulators and participate in creating sensible market practice; however, there can be no assurance as to when or if these matters will be clarified and a sensible market practice develop.
- 66 -
In other jurisdictions, security interests may be subject to enforcement and insolvency laws that differ significantly from those in Canada, and while the Company takes steps to understand the enforcement of security interests and the insolvency regimes of each jurisdiction in which it chooses to invest, the Group's security interests may not be enforceable and the application of such insolvency regimes as they apply to the Group's investments may remain unclear. Further, there can be no assurance that any judgments obtained in Canadian courts will be enforceable in any of those jurisdictions. If the Group is unable to enforce its security interests, there may be a material adverse effect on the Group.
COVID-19
The current global uncertainty with respect to the spread of the COVID-19, the rapidly evolving nature of the pandemic and local and international developments related thereto and its effect on the broader global economy and capital markets may impact the Group's business in the coming months.
The Group has taken proactive measures throughout the COVID-19 pandemic to protect the health and safety of its employees, to continue delivering high quality cannabis products to patients and to maintain its balance sheet.
While the precise impact of the COVID-19 outbreak on the Group remains unknown, rapid spread of COVID-19 and declaration of the outbreak as a global pandemic has resulted in travel advisories and restrictions, certain restrictions on business operations, social distancing precautions and restrictions on group gatherings which are having direct impacts on businesses in the State of Israel, Germany, Canada and around the world and could result in additional precautionary measures that could impact the Group's business. The spread of COVID-19 may also have a material adverse effect on global economic activity and could result in volatility and disruption to global supply chains and the financial and capital markets, which could interrupt supplies and other services from third parties upon which the Group relies, decrease demand for products, cause staff shortages, reduced customer traffic, and increased government regulation, all of which may cause a material adverse effect on the business, financial condition and results of operations of the Group.
Focus' Essential Service Designation
In response to the COVID-19 pandemic, the State of Israel has implemented, from time to time, mandatory shut-downs of non-essential businesses to prevent the spread of COVID-19. As of the date of this Annual Information Form, Focus has been deemed an "essential service", permitting it to continue production. Further public health measures or restrictions may require Focus to shut down or limit its operations in the State of Israel. Any disruptions to the business and operations of Focus in the event that Focus were to lose its designation as an "essential service" in the State of Israel may cause a material adverse effect on the business, financial condition and results of operations of the Group.
Conflicts of Interest
The Company may be subject to various potential conflicts of interest because of the fact that some of its officers and directors may be engaged in a range of business activities. In some cases, the executive officers and directors may have fiduciary obligations associated with these business interests that interfere with their ability to devote time to the Company and its affairs, and that could adversely affect Company operations. These business interests could require significant time and attention of the Company's executive officers and directors. In addition, the Company may also become involved in other transactions which conflict with the interests of the Company's directors and officers who may from time to time deal with persons, firms, institutions or corporations with which the Company may be dealing, or which may be seeking investments similar to those the Company desires. The interests of these persons could conflict with the Company's interests.
- 67 -
In addition, from time to time, these persons may be competing with the Company for available investment opportunities. Conflicts of interest, if any, will be subject to the procedures and remedies provided under applicable laws. In particular, in the event that such a conflict of interest arises at a meeting of directors, a director who has such a conflict will abstain from voting for or against the approval of such participation or such terms. In accordance with applicable laws, directors are required to act honestly, in good faith and in the Company's best interests.
Foreign Private Issuer Status under U.S. Securities Laws
The Company is a "foreign private issuer", under applicable U.S. federal securities laws, and is, therefore, not subject to the same requirements that are imposed upon U.S. domestic issuers by the SEC. Under the United States Securities Exchange Act of 1934, as amended (the "Exchange Act"), the Company is subject to reporting obligations that, in certain respects, are less detailed and less frequent than those of U.S. domestic reporting companies. As a result, the Company does not file the same reports that a U.S. domestic issuer would file with the SEC, although the Company is required to file with or furnish to the SEC the continuous disclosure documents that it is required to file in Canada under Canadian securities laws. In addition, the Company's officers, directors, and principal shareholders are exempt from the reporting and short-swing profit recovery provisions of Section 16 of the Exchange Act. Therefore, the Company's shareholders may not know on as timely a basis when the Company's officers, directors and principal shareholders purchase or sell Common Shares, as the reporting periods under the corresponding Canadian insider reporting requirements are longer.
As a foreign private issuer, the Company is exempt from the rules and regulations under the Exchange Act related to the furnishing and content of proxy statements. The Company is also exempt from Regulation FD, which prohibits issuers from making selective disclosures of material non-public information. While the Company complies with the corresponding requirements relating to proxy statements and disclosure of material non-public information under Canadian securities laws, these requirements differ from those under the Exchange Act and Regulation FD and shareholders should not expect to receive the same information at the same time as such information is provided by U.S. domestic companies. In addition, the Company may not be required under the Exchange Act to file annual and quarterly reports with the SEC as promptly as U.S. domestic companies whose securities are registered under the Exchange Act.
In addition, as a foreign private issuer, the Company has the option to follow certain Canadian corporate governance practices, except to the extent that such laws would be contrary to U.S. securities laws, and provided that the Company disclose the requirements it is not following and describe the Canadian practices it follows instead. The Company may in the future elect to follow home country practices in Canada with regard to certain corporate governance matters. As a result, the Company's shareholders may not have the same protections afforded to shareholders of U.S. domestic companies that are subject to all corporate governance requirements.
Loss of Foreign Private Issuer Status under U.S. Securities Laws
In order to maintain its status as a foreign private issuer, a majority of the Company's Common Shares must be either directly or indirectly owned by non-residents of the U.S. unless the Company also satisfies one of the additional requirements necessary to preserve this status. The Company may in the future lose its foreign private issuer status if a majority of its Common Shares are held in the U.S. and if the Company fails to meet the additional requirements necessary to avoid loss of its foreign private issuer status. The regulatory and compliance costs under U.S. federal securities laws as a U.S. domestic issuer may be significantly more than the costs incurred as a Canadian foreign private issuer eligible to use the multi-jurisdictional disclosure system adopted by the securities regulatory authorities in United States and Canada ("MJDS"). If the Company is not a foreign private issuer, it would not be eligible to use the MJDS or other foreign issuer forms and would be required to file periodic and current reports and registration statements on U.S. domestic issuer forms with the SEC, which are more detailed and extensive than the forms available to a foreign private issuer. In addition, the Company may lose the ability to rely upon exemptions from NASDAQ corporate governance requirements that are available to foreign private issuers.
- 68 -
Emerging Growth Company Status under U.S. Securities Laws
The Company is an "emerging growth company" as defined in section 3(a) of the Exchange Act (as amended by the JOBS Act, enacted on April 5, 2012), and the Company will continue to qualify as an emerging growth company until the earliest to occur of: (a) the last day of the fiscal year during which the Company has total annual gross revenues of US$1,070,000,000 (as such amount is indexed for inflation every five years by the SEC) or more; (b) the last day of the fiscal year of the Company following the fifth anniversary of the date of the first sale of common equity securities of the Company pursuant to an effective registration statement under the Securities Act, as amended; (c) the date on which the Company has, during the previous three year period, issued more than US$1,000,000,000 in non-convertible debt; and (d) the date on which the Company is deemed to be a "large accelerated filer", as defined in Rule 12b-2 under the Exchange Act. The Company will qualify as a large accelerated filer (and would cease to be an emerging growth company) at such time when on the last business day of its second fiscal quarter of such year the aggregate worldwide market value of its common equity held by non-affiliates will be US$700,000,000 or more.
For so long as the Company remains an emerging growth company, it is permitted to and intends to rely upon exemptions from certain disclosure requirements that are applicable to other public companies that are not emerging growth companies. These exemptions include not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act. The Company cannot predict whether investors will find the Common Shares less attractive because the Company relies upon certain of these exemptions. If some investors find the Common Shares less attractive as a result, there may be a less active trading market for the Common Shares and the Common Share price may be more volatile. On the other hand, if the Company no longer qualifies as an emerging growth company, the Company would be required to divert additional management time and attention from the Company's development and other business activities and incur increased legal and financial costs to comply with the additional associated reporting requirements, which could negatively impact the Company's business, financial condition and results of operations.
DIVIDENDS
As of the date of this Annual Information Form, the Company has not declared dividends on its Common Shares and has no intention to declare dividends on its Common Shares in the immediate or foreseeable future. There are no restrictions in the Company's articles or by-laws that prevent the Company from paying dividends. Any future dividends declared will made at the discretion of the Board and will depend on circumstances at the time of contemplation, including financial status of the Company, contractual or regulatory obligations, and other conditions existing at such future time.
- 69 -
DESCRIPTION OF CAPITAL STRUCTURE
Common Shares
The authorized capital of the Company consists of an unlimited number of Common Shares issuable in series which may contain the rights, privileges and restrictions as determined by the Board. Holders of Common Shares are entitled to dividends, if, as and when declared by the Board, to one vote per share at meetings of shareholders of the Company and, upon dissolution, to share equally in such assets of the Company as are distributable to the holders of Common Shares.
There are currently 50,500,985 Common Shares issued and outstanding as of the date of this Annual Information Form.
Warrants
In connection with the Reverse Takeover Transaction, a total of 9,730,258 Warrants were issued in exchange for the previously outstanding common share purchase warrants in the capital of a wholly-owned subsidiary of the Company. Prior to the Consolidation, each of these Warrants was exercisable by the holder thereof to acquire one Common Share at a price of $1.30 per Common Share until October 11, 2021.
As at the date of this Annual Information Form, the Company has 9,344,596 Warrants outstanding, including:
Broker Options
In connection with the Reverse Takeover Transaction, a total of 1,199,326 Broker Options, expiring on August 30, 2022, were issued in exchange for the previously outstanding broker compensation options in the capital of a wholly-owned subsidiary of the Company. Following the Consolidation, four Broker Options are required to be exercised to purchase one unit at an adjusted exercise price of $4.20, with each unit exercisable into one Common Share and one-half of one Warrant, with each whole Warrant expiring on August 30, 2022 and exercisable to purchase one Common Share at an exercise price of $5.20.
As of the date of this Annual Information Form, the Company has 674,414 Broker Options issued and outstanding.
Options
The Company has a stock option plan, as amended and restated on December 16, 2020 (the "Stock Option Plan") whereby a rolling maximum of 10% of the issued and outstanding Common Shares (less the number of Common Shares issuable pursuant to all other security based compensation arrangements such as the RSU Plan, as defined below under "Restricted Share Units") may be reserved for issuance pursuant to the exercise of Options (the "Option Cap"). The term of the Options granted are fixed by the Board, and are not to exceed 10 years. The exercise prices of the Options are determined by the Board, but shall not be less than the greater of the closing market price of the Common Shares on (a) the trading day prior to the date of grant of the Options; and (b) the date of grant of the Options.
- 70 -
The Options will vest as determined by the Board at the time of grant, except in a case relating to compensation for performing investor relations activities, in which case the Stock Option Plan requires the Options to vest in stages over 12 months with no more than one quarter of such Options vesting in any three month period. The maximum number of Common Shares reserved for issuance, pursuant to the Stock Option Plan and any other security based compensation arrangement of the Company, to (i) any one individual, in any 12 month period, is 5% of the total number of Common Shares then outstanding, unless approved by the disinterested shareholders; (ii) insiders (as a group), within a 12 month period, is 10% of the total number of Common Shares then outstanding, unless the Stock Option Plan approved by the disinterested shareholders; (iii) all investor relations persons, in a 12 month period, is 1% of the total number of Common Shares then outstanding; or (iv) any one consultant, in any 12 month period, is 2% of the total number of Common Shares then outstanding.
The Stock Option Plan was last approved by shareholders on December 16, 2020 and contains provisions for adjustment in the number of shares issuable thereunder in the event of a subdivision, consolidation, reclassification or change in the Common Shares, a merger or other relevant changes in the Company's capitalization. The Board may from time to time amend or reverse the terms of the Stock Option Plan or may terminate the Stock Option Plan at any time.
As at the date of this Annual Information Form, the Company had 3,582,389 Options outstanding with the material terms listed below.
|
Stock Option Grants |
|||
|
Date of Grant |
Number of Options |
Exercise Price |
Expiry Date |
|
October 11, 2019 |
792,475 |
$1.60 |
January 4, 2029 |
|
October 11, 2019 |
1,030,000 |
$1.60 |
September 11, 2029 |
|
October 11, 2019 |
50,000 |
$1.60 |
February 3, 2029 |
|
October 11, 2019 |
62,500 |
$1.60 |
April 7, 2029 |
|
October 11, 2019 |
2,500 |
$1.60 |
May 13, 2029 |
|
October 11, 2019 |
69,164 |
$1.60 |
August 11, 2029 |
|
October 11, 2019 |
37,500 |
$1.60 |
July 30, 2029 |
|
October 11, 2019 |
4,000 |
$4.20 |
October 9, 2022 |
|
June 9, 2020 |
590,000 |
$4.00 |
June 9, 2025 |
|
July 17, 2020 |
13,750 |
$5.80 |
July 17, 2025 |
|
October 23, 2020 |
28,750 |
$7.12 |
October 23, 2025 |
|
December 15, 2020 |
16,250 |
$8.56 |
December 15, 2025 |
|
February 8, 2021 |
5,500 |
$10.00 |
February 8, 2026 |
|
February 28, 2021 |
180,000 |
$10.00 |
February 28, 2026 |
|
March 18, 2021 |
700,000 |
$10.02 |
March 18, 2026 |
|
Total |
3,582,389 |
||
Restricted Share Units
On December 16, 2020, the Company's shareholders approved a restricted share unit plan ("RSU Plan") whereby the Company may issue restricted share units (each an "RSU") subject to a rolling maximum of 10% of the issued and outstanding Common Shares (less the number of Common Shares issuable pursuant to all other security based compensation arrangements such as the Stock Option Plan) that may be reserved for issuance pursuant to the exercise the RSUs. The RSU Plan supplements the Stock Option Plan by providing the Board with an alternative to issuing Options if, in the future, it determines that a full value share plan provides an attractive form of long-term incentive for key personnel provided that the aggregate Common Share issuances under the Stock Option Plan and RSU Plan combined does not exceed 10% of the Common Shares issued and outstanding (on a rolling basis).
- 71 -
The purpose of the RSU Plan is to provide a financial incentive for eligible employees, directors and consultants of the Company or an affiliate of the company to devote their best efforts to the long-term success of the Company's business, by aligning such participants' financial interests with those of the Company, to assist the Company in attracting and retaining individuals with top-level talent, passion and ability and to ensure that the total compensation provided to such eligible participants is at competitive levels.
RSUs will vest in such manner as determined by the Board or compensation committee of the Board at the time of grant. The maximum number of Common Shares reserved for issuance, pursuant to the RSU Plan and any other security based compensation arrangement of the Company, to (i) any one person, in a 12 month period, is 5% of the total number of Common Shares then outstanding, unless permitted by the CSE or approved by disinterested shareholders; (ii) insiders (as a group), at any time, is 10% of the total number of Common Shares then outstanding, unless permitted by the CSE or approved by disinterested shareholders; (iii) all investor relations persons, in a 12 month period, is 1% of the total number of Common Shares then outstanding; or (iv) any one consultant, in a 12 month period, is 1% of the total number of Common Shares then outstanding.
As of the date of this Annual Information Form, the Company has not issued any RSUs.
MARKET FOR SECURITIES
Trading Price and Volume
Common Shares
The Common Shares have been listed for trading under the symbol "IMCC" on the CSE since November 5, 2019, following the completion of the Reverse Takeover Transaction, and on the NASDAQ since March 1, 2021. The chart below sets out the monthly trading history of the Common Shares on the CSE for the financial year ended December 31, 2020.
| Common Share Historic Trading Prices and Volumes | |||
| Month | High (CAD) |
Low (CAD) |
Volume |
| December | 3.50 | 2.00 | 2,023,546 |
| November | 2.30 | 1.55 | 3,057,100 |
| October | 1.84 | 1.20 | 3,407,549 |
| September | 1.59 | 1.05 | 1,247,607 |
| August | 1.69 | 1.25 | 4,078,659 |
| July | 1.75 | 1.16 | 5,166,395 |
| June | 1.34 | 0.65 | 6,013,936 |
| May | 0.82 | 0.51 | 9,189,313 |
| April | 0.65 | 0.20 | 6,123,027 |
| March | 0.35 | 0.175 | 159,774 |
| February | 0.475 | 0.30 | 127,419 |
| January | 0.51 | 0.34 | 3,035,358 |
Notes:
(1) All trading prices and volumes in this table are provided on a pre-Consolidation basis.
- 72 -
Warrants
The Warrants are listed for trading on the CSE under the symbol "IMCC.WT" until their expiry date of October 11, 2021. The below trading information chart sets out the monthly trading history of the Warrants on the CSE for the financial year ended December 31, 2020.
|
Warrants Historic Trading Prices and Volumes |
|||
|
Month |
High (CAD) |
Low (CAD) |
Share Volume |
|
December |
1.78 |
1.00 |
291,819 |
|
November |
1.19 |
0.47 |
100,740 |
|
October |
0.72 |
0.23 |
501,949 |
|
September |
0.60 |
0.25 |
142,119 |
|
August |
0.60 |
0.50 |
23,100 |
|
July |
0.55 |
0.20 |
176,000 |
|
June |
0.35 |
0.06 |
185,332 |
|
May |
0.095 |
0.04 |
288,500 |
|
April |
0.05 |
0.005 |
666,500 |
|
March |
- |
- |
0 |
| February | - | - | 0 |
| January | - | - | 0 |
Notes:
(1) No Warrants listed on the CSE were traded in January through March 2020
- 73 -
Prior Sales
The table below summarizes details of securities of the Company that were not listed or quoted on a marketplace and issued by the Company during the financial year ended December 31, 2020. For a list of all outstanding options granted as of the date of this Annual Information Form, please see "Description of Capital Structure - Options" above.
|
Prior Sales of Unlisted or Unquoted Securities |
|||
|
Date of Issuance |
Security |
Issuance/Exercise Price Per Security (CAD)(1) |
Number of Securities(1) |
|
April 30, 2020 |
Unlisted Warrants(2) |
0.50 |
86,550 |
|
May 7, 2020 |
Unlisted Warrants(2) |
0.50 |
171,625 |
|
June 3, 2020 |
Unlisted Warrants(2) |
0.50 |
211,095 |
|
June 5, 2020 |
Unlisted Warrants(2) |
0.50 |
21,875 |
|
June 5, 2020 |
Unlisted Warrants(2) |
0.50 |
31,370 |
|
June 9, 2020 |
Options(4) |
1.00 |
2,965,000 |
|
June 26, 2020 |
Unlisted Warrants(2) |
0.50 |
70,745 |
|
July 17, 2020 |
Options(5) |
1.45 |
105,000 |
|
July 28, 2020 |
Unlisted Warrants(2) |
1.30 |
129,815 |
|
October 23, 2020 |
Options(6) |
1.78 |
130,000 |
|
November 5, 2020 |
Unlisted Warrants(2) |
1.30 |
19,362 |
|
December 3, 2020 |
Unlisted Warrants(2) |
1.30 |
14,462 |
|
December 15, 2020 |
Options(7) |
2.14 |
70,000 |
Notes:
(1) Figures are reported on a pre-Consolidation basis.
(2) During the financial year ended December 31, 2020, the Company issued an aggregate of 756,899 Unlisted Warrants pursuant to exercises of Broker Options. As of the date of this Annual Information Form, all Unlisted Warrants issued during the financial year ended December 31, 2020 have either expired or been exercised.
(3) Following the Consolidation, all Options outstanding issued prior to February 12, 2021 were consolidated on the basis of four (4) pre-Consolidation Options to one (1) post-Consolidation Option (a "Post-Consolidation Option"), with respective exercise prices adjusted upwards by a factor of four (4). Each Post-Consolidation Option is exercisable for one Common Share at the adjusted exercise price.
(4) Each Option is exercisable at the exercise price for one Common Share and expires on June 9, 2025. As of the date of this Annual Information Form, 590,000 of these Options, on a post-Consolidation basis, remain outstanding.
(5) Each Option is exercisable at the exercise price for one Common Share and expires on July 17, 2025. As of the date of this Annual Information Form, 13,750 of these Options on a post-Consolidation basis, remain outstanding.
(6) Each Option is exercisable at the exercise price for one Common Share and expires on October 23, 2025. As of the date of this Annual Information Form, 28,750 of these Options on a post-Consolidation basis, remain outstanding.
(7) Each Option is exercisable at the exercise price for one Common Share and expires on December 15, 2025. As of the date of this Annual Information Form, 16,250 of these Options, on a post-Consolidation basis, remain outstanding.
- 74 -
ESCROWED SECURITIES AND SECURITIES SUBJECT TO
CONTRACTUAL RESTRICTIONS ON TRANSFER
As of December 31, 2020, none of the Company's securities of any class are subject to a contractual restriction or are being held in escrow.
DIRECTORS AND EXECUTIVE OFFICERS
The following table sets out the name, province or state, and country of residence, positions and offices held with the Company, the period during which each director has served as a director and the principal occupations of each of the directors and executive officers as of the date hereof. Directors of the Company hold office until the next annual meeting of shareholders or until their successors are duly elected or appointed, unless his office is earlier vacated in accordance with the Company's articles or by-laws:
|
Director and Executive Officer Information |
|||
|
Name and
|
Office with Company
|
Principal Occupation and Positions
|
Number and |
|
Oren Shuster(6) Ra'anana, Israel
|
Chief Executive Officer and Director since October 2019 |
CEO of IMC Holdings since 2008; Co-CEO of Ewave Group Ltd. since 1999. |
9,135,137(7) (18.09%) |
|
Shai Shemesh Petach-Tikva, Israel
|
Chief Financial Officer since October 2019 |
CFO of IMC Holdings since 2019; CFO of Sadyt Israel and IVM Minrav-Sadyt from 2011 to 2019. |
11,905 |
|
Yael Harrosh Tel Aviv, Israel
|
General Counsel and Corporate Secretary since October 2019 |
General Counsel, Corporate Secretary and Business and Compliance Manager of IMC Holdings since 2018; Legal Counsel and Deputy CEO at ProMarket Group from 2016 to 2018; Advocate at AYR Law Firm, Israel from 2015 to 2016. |
Nil |
|
Haleli Barath(4)(5) Tel Aviv, Israel
|
Director since February 2021 |
Partner of Bfp & Co. since 2009. |
166,682 (<1%) |
|
Vivian Bercovici(4)(5)(6) Tel Aviv, Israel |
Director since March 2020 |
Independent consultant and columnist, Managing Director, Europe and Israel at Nuuvera Inc. from 2017 to 2018; Canadian Ambassador to Israel from 2014 to 2016. |
Nil |
|
Brian Schinderle(4)(5) Illinois, USA |
Director since February 2021 |
Founder and Manager of Solidum Capital since 2017; EVP-Finance of GHG Management, DBA Grassroots Cannabis from 2018 to 2020; Portfolio Manager of Balyasny Asset Management from 2009 to 2017. |
Nil |
|
Marc Lustig(6) West Vancouver, British Columbia |
Director since October 2019, Executive Chairman since December 2020 and Chairman from October 2019 to December 2020 |
Director of Pharmacielo Ltd. since November 2020; Director of Cresco Labs Inc. since June 2020; Director of Trichome Financial Corp. since October 2019; Founder, Chairman and Chief Executive Officer of CannaRoyalty Corp. (dba Origin House) from 2016 to 2020. |
761,839 (1.51%) |
(1) Assumes 50,500,985 Common Shares issued and outstanding.
(2) Does not include the 6,174,418 Common Shares issuable on the full exercise of 3,582,389 outstanding Options, 9,344,596 outstanding Warrants and 674,414 outstanding Broker Options, including the 84,302 Common Shares issuable upon exercise of the underlying Warrants issued upon exercise of such Broker Options.
(3) As of the date hereof, all directors and executive officers noted above of the Company, as a group, beneficially own, directly or indirectly, or exercise control or direction over 10,075,563 Common Shares of the Company, representing 19.95% of the Company's outstanding Common Shares.
(4) Member of the Audit Committee.
(5) Member of the Compensation Committee.
(6) Member of the Governance and Nomination Committee.
(7) 9,133,602 shares are held directly by Oren Shuster and 1,535 shares are held by Ewave Group Ltd., an entity of which Mr. Shuster owns and controls 50% of the outstanding ordinary shares.
- 75 -
Cease Trade Orders, Bankruptcies, Penalties or Sanctions
To the knowledge of the Company, no director or executive officer of the Company is, as at the date of this Annual Information Form, or has been, within the 10 years before the date of this Annual Information Form, a director, chief executive officer or chief financial officer of any company (including the Company) that:
(a) was the subject of a cease trade or similar order or an order that denied the relevant company access to any exemption under securities legislation, for a period of more than 30 consecutive days that was issued while the director or executive officer was acting in the capacity as director, chief executive officer or chief financial officer; or
(b) was subject to a cease trade or similar order or an order that denied the relevant company access to any exemption under securities legislation, for a period of more than 30 consecutive days that was issued after the director or executive officer ceased to be a director, chief executive officer or chief financial officer and which resulted from an event that occurred while that person was acting in the capacity as director, chief executive officer or chief financial officer,
To the knowledge of the Company, no director or executive officer of the Company, or a shareholder holding a sufficient number of securities of the Company to affect materially the control of the Company:
(a) is, as at the date of this Annual Information Form, or has been within the 10 years before the date of the Annual Information Form, a director or executive officer of any company (including the Company) that, while that person was acting in that capacity, or within a year of that person ceasing to act in that capacity, became bankrupt, made a proposal under any legislation relating to bankruptcy or insolvency or was subject to or instituted any proceedings, arrangement or compromise with creditors or had a receiver, receiver manager or trustee appointed to hold its assets; or
(b) has, within the 10 years before the date of this Annual Information Form, become bankrupt, made a proposal under any legislation relating to bankruptcy or insolvency, or become subject to or instituted any proceedings, arrangement or compromise with creditors, or had a receiver, receiver manager or trustee appointed to hold the assets of the director, executive officer or shareholder.
- 76 -
To the knowledge of the Company, no director or executive officer of the Company, or a shareholder holding a sufficient number of securities of the Company to affect materially the control of the Company, has been subject to:
(a) any penalties or sanctions imposed by a court relating to securities legislation or by a securities regulatory authority or has entered into a settlement agreement with a securities regulatory authority; or
(b) any other penalties or sanctions imposed by a court or regulatory body that would likely be considered important to a reasonable investor in making an investment decision.
Conflicts of Interest
There are potential conflicts of interest to which the directors, officers and promoters of the Company will be subject with respect to the operations of the Company. Certain directors and/or officers serve as directors and/or officers of other companies or have significant shareholdings in other companies. Situations may arise where the directors, officers and promoters of the Company will be engaged in direct competition with the Company. Any conflicts of interest will be subject to and governed by the law applicable to directors and officers conflicts of interest, including the procedures prescribed by the BCBCA. The BCBCA requires that directors and officers of the Company, who are also directors or officers of a party which enters into a material contract with the Company or otherwise have a material interest in a material contract entered into by the Company, must disclose their interest and, in certain instances, refrain from voting on any resolution of the Company's directors to approve the contract.
PROMOTERS
Oren Shuster, CEO and director of the Company and Rafael Gabay, a consultant and former director of the Company, may be considered to be promoters because they founded and organized the business of IMC Holdings prior to the Reverse Takeover Transaction. Mr. Shuster is a resident of Ra'anana, Israel and controls 9,135,137 Common Shares, representing 18.09% of the issued and outstanding Common Shares on a non-diluted basis. Mr. Gabay is a resident of Ganot, Israel and controls 8,090,720 Common Shares, representing 16.02% of the issued and outstanding Common Shares on a non-diluted basis. 9,133,602 Common Shares and 8,089,185 Common Shares are held directly by Oren Shuster and Rafael Gabay, respectively, and 1,535 Common Shares are owned by Ewave Group Ltd., an entity which is jointly owned and controlled by Messrs. Shuster and Gabay.
Under the IMC Restructuring, IMC Holdings sold its interest in Focus to Messrs. Shuster and Gabay and retained options to re-acquire these entities pursuant to the Focus Agreement as described above in "Corporate Structure - Intercorporate Relationships".
LEGAL PROCEEDINGS AND REGULATORY ACTIONS
Legal Proceedings
Except for the proceedings disclosed below pertaining to Focus, there are no actual or pending material legal proceedings to which the Group or any of its subsidiaries or affiliates are a party or of which any of their assets are subject. Management of the Company is not aware of any such material legal proceedings contemplated.
- 77 -
Class Action T.Z. 35676-08-19
On August 19, 2019, a motion was filed for approval of a class action (the "Motion") against 17 companies (the "Parties") operating in the field of medical cannabis in Israel, including Focus. The applicant's argument is that the Parties did not accurately mark the concentration of active ingredients in their products. The personal suit sum for each class member stands at NIS 15,585 and the total amount of the class action suit is estimated at NIS 685,740,000. On June 2, 2020, the Parties submitted their response to the Motion. The Parties argue in their response that the threshold conditions for approval of a class action were not met, since there is no reasonable possibility that the causes of action in the Motion will be decided in favor of the class group. On July 3, 2020 the applicant submitted his response to the Parties' response. On July 5, 2020 the applicant was absent from the hearing. As a result, on July 23, 2020 the Parties filed an application for a ruling of expenses which received a response from the applicant on August 12, 2020, asking to decline this request. On September 29, 2020 the court ruled that the applicant would pay the Parties' expenses amount of NIS 750. Prehearing is set for July 14, 2021.
As of the date of this Annual Information Form, based on the current preliminary state of the litigation process, the Company's management believes that it is not reasonably possible to assess the outcome of the proceeding.
Supreme Court of Justice 2335/19
On October 6, 2019, Focus received a decision regarding a petition that was filed against the MOH, concerning the new regulatory framework of the cannabis market and demanding that the court resolve as follows:
that the MOH immediately suspend the implementation of the new regulation that harms, disproportionally, the medical cannabis patients;
that the implementation of the new regulation, as is, would cause violation of constitutional rights of the medical cannabis patients; and
that the MOH amends the flaws of the new regulation, prior to becoming effective, and to establish new regulations regarding labeling and use of pesticides.
The decision provided for an interim injunction, extending the validity of patient licenses until the earlier of March 31, 2020 or 10 days after the date the MOH reaches a conclusion regarding the price control of medical cannabis products.
According to the decision, Focus was attached to the proceedings as a respondent. Accordingly, Focus filed its response to the petition on November 12, 2019.
On March 8, 2020, the court decided to extend the validity of the interim injunction, so that the medical cannabis use licenses, which were extended under the decision, would continue to be valid until May 15, 2020, or 10 days after the price committee's decision on the matter before it, whichever comes first, subject to another court decision.
The court also decided that if a further extension of the period of the interim injunction is granted beyond May 15, 2020, to the extent required, it would be subject to medical surveillance by the attending physician, that his details of which were to be included in the patient's existing use license.
- 78 -
In light of several applications by the respondent represented by the state attorney's office, for extension to file updated notice to the court, the interim injunction was extended on July 30, 2020, until and subject to other decision of the court.
On October 29, 2020, the respondents represented by the state attorney's office filed an update notice stating that the appeals committee unanimously decided against imposing price controls on medical cannabis products and that the prices committee would hold a follow-up hearing in four months. The respondents also requested to update the court again in two months.
On November 25, 2020, the petitioner submitted their response to the respondents' update.
On March 25, 2021, the respondents represented by the State Attorney's Office filed an updating notice stating that the Prices Committee had come to a decision against imposing price controls on medical cannabis products. However, the Prices Committee announced that it will issue an RFI to the corporations engaged in the medical cannabis market and assess the market every six months. Following the aforementioned, the respondents represented by the State Attorney's Office believe that the appeal should be rejected and the interim injunction should be canceled. On April 13, 2021, three of the respondents filed a response to the court, requesting to reject the appeal and to cancel the interim injunction.
As of the date of this Annual Information Form, based on Focus' legal counsel opinion, the Company's management believes that the chances of the petition are less than 50%.
Regulatory Actions
There have not been any penalties or sanctions imposed against the Company by a court relating to provincial or territorial securities legislation or by a securities regulatory authority, nor have there been any other penalties or sanctions imposed by a court or regulatory body against the Company that would likely be considered important to a reasonable investor in making an investment decision, and the Company has not entered into any settlement agreements before a court relating to provincial or territorial securities legislation or with a securities regulatory authority.
INTEREST OF MANAGEMENT AND OTHERS IN MATERIAL TRANSACTIONS
Except as described below and otherwise disclosed in this Annual Information Form, to the Company's knowledge, no director or executive officer of the Company or any person or company that is the direct or indirect beneficial owners of, or who exercises control or direction over, more than 10% of any class of the Company's outstanding voting securities, or an associate or affiliate of any persons or companies referred to in this paragraph, has any material interest, direct or indirect, in any transaction within the financial years ended December 31, 2020, December 31, 2019, April 30, 2019 or as of the date of this Annual Information Form, or in any proposed transaction, that has materially affected or will materially affect the Company.
At the time of voting for the Trichome Transaction by the Company's board of directors, Marc Lustig, the executive chairman and a director of the Company, was also a director of Trichome. Accordingly, Mr. Lustig had a disclosable interest with respect to the Trichome Transaction and, in accordance with Canadian corporate law requirements, he declared the nature and extent of his interest in the Trichome Transaction and recused himself from consideration and voting on the Trichome Transaction as a director. As of the date of this Annual Information Form, Mr. Lustig continues to serve as executive chairman and director of the Company and as a director of Trichome.
- 79 -
TRANSFER AGENTS AND REGISTRARS
The transfer agent and registrar for the Common Shares and warrant agent for the Listed Warrants is Computershare Investor Services Inc., located at 510 Burrard Street, 3rd Floor, Vancouver, British Columbia V6C 3B9. The United States co-transfer agent of the Company is Continental Stock Transfer & Trust Company, located at 1 State Street, 30th Floor, New York, NY 10004-1561.
MATERIAL CONTRACTS
Except for contracts entered into in the ordinary course of business, the only material contracts the Company or a subsidiary is a party to as the date of this Annual Information Form are the following:
the IP Agreement dated April 2, 2019, between IMC Holdings and Focus, as further described in "Corporate Structure - Intercorporate Relationships";
the Services Agreement dated April 2, 2019, between IMC Holdings and Focus, as further described in "Corporate Structure - Intercorporate Relationships";
the Focus Agreement dated April 2, 2019, between IMC Holdings, Oren Shuster and Rafael Gabay, as further described in "Corporate Structure - Intercorporate Relationships";
the warrant indenture dated August 30, 2019 between the Company and Computershare Trust Company of Canada (the "Warrant Indenture") entered into in connection with the issuance of Warrants in connection with the Reverse Takeover Transaction;
the supplemental warrant indenture dated November 14, 2019 between the Company and Computershare Trust Company of Canada amending the Warrant Indenture entered into in connection with the re-issuance of Warrants and the Reverse Takeover Transaction;
the arrangement agreement dated December 30, 2020, as subsequently amended on January 22, 2021 and March 14, 2021, between the Company and Trichome entered into in connection with the Trichome Transaction; and
the arrangement agreement dated March 31, 2021 between the Company, Trichome and MYM entered into in connection with the MYM Transaction.
Copies of the above material contracts are available on the Company's SEDAR profile at www.sedar.com.
INTERESTS OF EXPERTS
Names of Experts
The following are the persons or companies who were named as having prepared or certified a statement, report, opinion or valuation described or included in a filing, or referred to in a filing, made under National Instrument 51-102 - Continuous Disclosure Obligations by the Company during, or relating to, the financial year ended December 31, 2020, and whose profession or business gives authority to the statement, report, valuation, or opinion made by the person or company:
The annual consolidated financial statements as of December 31, 2020, audited by Kost Forer Gabbay & Kasierer, a member of Ernst & Young Global, an independent registered public accounting firm, have been included in reliance on their report given on their authority as experts in accounting and auditing.
- 80 -
Interests of Experts
Kost Forer Gabbay & Kasierer, a member of Ernst & Young Global, have confirmed that they are independent with respect to the Company within the meaning of the U.S. Securities Act of 1933 (the "Securities Act") and the applicable rules and regulations thereunder adopted by the SEC and the Public Company Accounting Oversight Board (United States).
AUDIT COMMITTEE INFORMATION
Audit Committee Charter
The charter of the Company's Audit Committee is attached to this Annual Information Form as Schedule "A".
Composition of Audit Committee
As of the date of this Annual Information Form, the members of the Audit Committee are Haleli Barath, Brian Schinderle (Chair) and Vivian Bercovici, all of whom are "independent", and all of whom are "financially literate" as such terms are defined in National Instrument 52-110 - Audit Committees.
Each of the Audit Committee members has an understanding of the accounting principles used to prepare the Company's financial statements, experience preparing, auditing, analyzing or evaluating comparable financial statements and experience as to the general application of relevant accounting principles, as well as an understanding of the internal controls and procedures necessary for financial reporting.
The Audit Committee has the primary function of fulfilling its responsibilities in relation to reviewing the integrity of the Company's financial statements, financial disclosures and internal controls over financial reporting; monitoring the system of internal control; monitoring the Company's compliance with legal and regulatory requirements, selecting the external auditor for shareholder approval; reviewing the qualifications, independence and performance of the external auditor; and reviewing the qualifications, independence and performance of the Company's internal auditors. The Audit Committee has specific responsibilities relating to the Company's financial reports; the external auditor; the internal audit function; internal controls; regulatory reports and returns; legal or compliance matters that have a material impact on the Company; and the Company's whistleblowing procedures. In fulfilling its responsibilities, the Audit Committee meets regularly with the internal and external auditor and key management members. The full text of the Audit Committee's charter is disclosed in Appendix "A".
Relevant Experience and Education
Brian Schinderle
Mr. Schinderle is the Founder and Managing Partner of Solidum Capital Advisors LLC ("Solidum"). Solidum invests its own capital and works in a merchant banking and advisory capacity with a select group of companies in the cannabis sector. In addition, from 2018 to 2020, Mr. Schinderle served as Executive Vice President -Finance of GR Companies Inc. (dba Grassroots Cannabis) ("Grassroots"), focusing on finance, strategy, capital markets, investor relations, mergers and acquisitions. In July 2020, Grassroots merged with Curaleaf Holdings, Inc. (CSE:CURA) in a transaction valued at approximately US$850million. Prior to forming Solidum in 2017, Mr. Schinderle spent over 20 years in investment management, primarily investing in fixed income and equity assets via hedge funds, private equity and discretely managed funds. Mr. Schinderle currently serves on the advisory boards of Altitude Investments Inc.and AIM PLC, as well as the Board of Directors of Bazelet Americas, LLC.
- 81 -
Haleli Barath
Ms. Barath is the Co-founder and Senior Partner at BFP & Co., an Israel-based law firm. Ms. Barath has over 20 years' experience advising Israeli and international corporations on a wide range of sophisticated cross-border and domestic transactions. Ms. Barath advises Fortune 500 international corporations, funds and prominent early-stage start-ups and growth companies in Israel on a range of sectors including enterprise software, cybersecurity, fintech, biotech, cannabis and digital health and is an active partner in their development and growth. Ms. Barath is also the Co-founder and General Partner of Cerca Partners, a venture capital firm that invests in Israeli technology companies. Ms.Barath holds an LLB degree from the Hebrew University in Israel, is a member of the Israeli bar and lectures at universities and various business forums on topics ranging from corporate and business law to technology and regulatory matters.
Vivian Bercovici
Ms. Bercovici is currently a consultant to various medical cannabis and medical device entities regarding operational issues and market opportunities in Israel, Europe and Canada. Previously, from March 2017 to March 2018, Ms. Bercovici was the Managing Director, Israel and Europe operation at Nuuvera Inc., a Toronto-based medical cannabis company that was acquired by Aphria Inc. Also, Ms. Bercovici served as Canadian Ambassador to Israel from 2014 to 2016, having been appointed by then Prime Minister Stephen Harper. Ms. Bercovici has vast experience of over 20 years practicing law in Toronto, specializing in media defence and financial services regulatory law. Ms. Bercovici holds a Bachelor of Arts from York University, a Postgraduate Diploma in International Relations from the London School of Economics and Political Science and a Bachelor of Laws from the University of Toronto.
Audit Committee Oversight
At no time since the commencement of the financial year ended December 31, 2020 was a recommendation of the Audit Committee to nominate or compensate an external auditor not adopted by the Board.
Reliance on Certain Exemptions
At no time since the commencement of the financial year ended December 31, 2020 has the Company relied on the exemption in:
(a) Section 2.4 of NI 52-110 (De Minimis Non-audit Services);
(b) Subsection 6.1.1(4) of NI 52-110 (Circumstances Affecting the Business or Operations of the Venture Issuer);
(c) Subsection 6.1.1(5) of NI 52-110 (Events Outside Control of Member);
(d) Subsection 6.1.1(6) of NI 52-110 (Death, Incapacity or Resignation); or
(e) an exemption from NI 52-110, in whole or in part, granted under Part 8 of NI 52-110.
The Company is relying on the exemption provided in Section 6.1 of NI 52-110 as the Company was a "venture issuer" as at December 31, 2020. As a result, the Company is exempt from the requirements of Part 3 (Composition of Audit Committee) and Part 5 (Reporting Obligations) of NI 52-110.
Pre-Approval Policies and Procedures
The Audit Committee has adopted specific policies and procedures for the engagement of non-audit services as described in Schedule "A" attached hereto.
- 82 -
External Auditor Service Fees (by Category)
Audit Fees
Jackson and Company, Chartered Accountants served as the Company's external auditors between February 1, 2017 and January 21, 2019. Dale Matheson Carr-Hilton Labonte LLP served as the Company's external auditors between January 21, 2019 and January 16, 2020 and was immediately succeeded by Kost Forer Gabbay & Kasierer, a member of Ernst & Young Global. The following table lists the aggregate fees paid or payable to all external auditors, by category, for the financial years ended December 31, 2020 and December 31, 2019:
|
|
December 31, 2020(1) |
December 31, 2019(1) |
|
Audit fees(2) |
$239,308 |
$120,168 |
|
Audit-related fees(3) |
- |
- |
|
Tax fees(4) |
$4,074 |
- |
|
All other fees(5) |
- |
- |
|
Total fees |
$243,382 |
$120,168 |
(1) Amounts are stated in USD.
(2) Audit fees consist of the aggregate fees billed for the audit or review of the Company's annual financial statements that are normally provided in connection with statutory and regulatory filings or engagements.
(3) Audit-related fees consist of the aggregate fees billed for assurance and related services that are reasonably related to the performance of the audit or review of the Company's financial statements and are not reported as audit fees.
(4) For tax compliance, tax advice and tax planning.
(5) For products and services other than the audit fees, audit-related fees and tax fees described above.
ADDITIONAL INFORMATION
Additional information relating to the Company may be found on SEDAR at www.sedar.com. Additional information, including directors' and officers' remuneration and indebtedness, principal holders of the Company's securities and securities authorized for issuance under equity compensation plans are contained in the Company's information circular for its most recent annual meeting of shareholders held on March 16, 2020. Additional information is also provided in the Company's financial statements and MD&A for the financial year ended December 31, 2020.
SCHEDULE "A"
IM CANNABIS CORP.
AUDIT COMMITTEE CHARTER
PURPOSE OF THE COMMITTEE
The purpose of the Audit Committee (the "Committee") of the Board of Directors (the "Board") of IM Cannabis Corp. (the "Company") is to provide an open avenue of communication between management, the Company's independent auditor and the Board and to assist the Board in its oversight of:
(a) the integrity, adequacy and timeliness of the Company's financial reporting and disclosure practices;
(b) the Company's compliance with legal and regulatory requirements related to financial reporting; and
(c) the independence and performance of the Company's independent auditor.
The Committee shall also perform any other activities consistent with this audit committee charter (the "Charter"), the Company's articles, the rules and regulations of all exchanges on which the securities of the Company are listed for trading, National Instrument 52-110 - Audit Committees, as amended from time to time ("NI 52-110"), the Business Corporations Act (British Columbia), the United States Securities Exchange Act of 1934 (the "Exchange Act"), as amended for issuers listed on the NASDAQ Capital Market ("NASDAQ") and any other applicable laws as required or deemed necessary or appropriate by the Committee or Board (collectively, the "Applicable Laws").
The Committee's role is one of oversight of the conduct of those activities by the Company's management and external auditors, including oversight of the accounting and financial reporting processes of the Company and the audits of the financial statements of the Company. Management is responsible for preparing the Company's financial statements and other financial information and for the fair presentation of the information set forth in the financial statements in accordance with international financial reporting standards ("IFRS"). Management is also responsible for establishing internal controls and procedures and for maintaining the appropriate accounting and financial reporting principles and policies designed to assure compliance with accounting standards and all Applicable Laws.
The independent auditor's responsibility is to audit the Company's financial statements and provide its opinion, based on its audit conducted in accordance with generally accepted auditing standards, that the financial statements present fairly, in all material respects, the financial position, results of operations and cash flows of the Company in accordance with IFRS.
The Committee is responsible for recommending to the Board the independent auditor to be nominated for the purpose of auditing the Company's financial statements, preparing or issuing an auditor's report or performing other audit, review or attest services for the Company, and for reviewing and recommending the compensation of the independent auditor. The Committee is also directly responsible for the evaluation of and oversight of the work of the independent auditor.
The independent auditor shall report directly to the Committee.
COMMITTEE RESPONSIBILITIES
In addition to the foregoing, in performing its oversight responsibilities the Committee shall:
1. Have the funding and authority to discharge its duties and responsibilities.
- 2 -
2. Monitor the adequacy of this Charter on an annual basis and recommend any proposed changes to the Board.
3. Review the appointments of the Company's Chief Financial Officer and any other key financial executives involved in the financial reporting process.
4. Review with management and the independent auditor the adequacy and effectiveness of the Company's accounting and financial controls and the adequacy and timeliness of its financial reporting processes.
5. Review with management and the independent auditor the annual financial statements and related documents and review with management the unaudited quarterly financial statements and related documents, prior to filing or distribution, including matters required to be reviewed under applicable legal or regulatory requirements.
6. Where appropriate and prior to release, review with management any news releases that disclose annual or interim financial results or contain other significant financial information that has not previously been released to the public.
7. Review the Company's financial reporting and accounting standards and principles and significant changes in such standards or principles or in their application, including key accounting decisions affecting the financial statements, alternatives thereto and the rationale for decisions made.
8. Review the quality and appropriateness of the accounting policies and the clarity of financial information and disclosure practices adopted by the Company, including consideration of the independent auditor's judgment about the quality and appropriateness of the Company's accounting policies. This review may include discussions with the independent auditor without the presence of management.
9. Review with management and the independent auditor significant related party transactions and potential conflicts of interest.
10. Pre-approve all non-audit services to be provided to the Company by the independent auditor.
11. Ensure receipt from the independent auditors of a formal written statement delineating all relationships between the auditor and the Company, and monitor the independence of the independent auditor by reviewing all relationships between the independent auditor and the Company and all non-audit work performed for the Company by the independent auditor and to engage in a dialogue with the independent auditors with respect to any disclosed relationships or services that may impact the objectivity and independence of the external auditors.
12. Establish and review the Company's procedures for the:
• receipt, retention and treatment of complaints regarding accounting, financial disclosure, internal controls or auditing matters; and
• confidential, anonymous submission by employees regarding questionable accounting, auditing and financial reporting and disclosure matters.
13. Conduct or authorize investigations into any matters that the Committee believes is within the scope of its responsibilities.
14. Perform such other functions and exercise such other powers as are prescribed or required by the articles of the Company or pursuant to Applicable Laws as set out for the audit committee of a reporting issuer under NI 52-110, section 224 of the Business Corporations Act (British Columbia) and the Exchange Act.
- 3 -
DIRECTORS MAY REQUEST MEETING
Any Director of the Company may request the chair of the Committee (the "Chair") to call a meeting of the Committee and may attend at such meeting or inform the Committee of a specific matter of concern to such Director, and may participate in such meeting to the extent permitted by the Chair.
The times of and places where the meetings of the Committee shall be held and the calling of and procedure at such meetings shall be determined from time to time by the Committee.
COMMITTEE STRUCTURE AND AUTHORITY
(a) Composition
The Committee shall consist of at least three directors as determined by the Board, all of whom shall qualify as independent directors pursuant to (i) NI 52-110; (ii) Rule 5605 of the NASDAQ Stock Market Rules; (iii) Rule10A-3(b)(1) under the Exchange Act; and (iv) any additional requirements or guidelines for audit committee service under applicable securities laws and the rules of any stock exchange on which the shares of the Company are listed for trading.
All members of the Committee shall be financially literate, as defined in NI 52-110, and at least one member shall have "accounting or related financial management expertise". In particular, at least one member shall have: (i) education and experience as a principal financial officer, principal accounting officer, controller, public accountant or auditor or experience in one or more positions that involve the performance of similar functions; (ii) experience actively supervising a principal financial officer, principal accounting officer, controller, public accountant, auditor or person performing similar functions; (iii) experience overseeing or assessing the performance of companies or public accountants with respect to the preparation, auditing or evaluation of financial statements; or (iv) other relevant experience:
(i) an understanding of generally accepted accounting principles and financial statements;
(ii) the ability to assess the general application of such principles in connection with the accounting for estimates, accruals and provisions;
(iii) expertise preparing, auditing, analyzing or evaluating financial statements that present a breadth and level of complexity of accounting issues that are generally comparable to the breadth and complexity of issues that can reasonably be expected to be raised by the Company's financial statements, or experience actively supervising one or more persons engaged in such activities;
(iv) an understanding of internal controls and procedures for financial reporting; and
(v) an understanding of audit committee functions.
Committee members may not, other than in their respective capacities as members of the Committee, the Board or any other committee of the Board, accept directly or indirectly any consulting, advisory or other compensatory fee from the Company or any subsidiary of the Company, or be an "affiliated person" (as such term is defined in the Exchange Act and the rules adopted by the U.S. Securities and Exchange Commission thereunder) of the Company or any subsidiary of the Company. For greater certainty, directors' fees and fixed amounts of compensation under a retirement plan (including deferred compensation) for prior service with the Company that are not contingent on continued service should be the only compensation an audit committee member may receive from the Company.
- 4 -
No Committee member shall serve on the audit committees of more than three other issuers without prior determination by the Board that such simultaneous service would not impair the ability of such member to serve effectively on the Committee.
(b) Appointment of Replacement Committee Members
Each member of the Committee shall serve at the pleasure of the Board. Any member of the Committee may be removed or replaced at any time by the Board and shall automatically cease to be a member of the Committee upon ceasing to be a Director of the Company.
The Board may fill vacancies on the Committee by appointment from amongst its number. The Board shall fill any vacancy if the membership of the Committee is less than three directors. If and whenever a vacancy shall exist on the Committee, the remaining members may exercise all their power so long as a quorum remains in office.
Subject to the foregoing, the members of the Committee shall be appointed by the Board annually and each member of the Committee shall hold office until the next annual meeting of the shareholders of the Company after his or her election or until his or her successor shall be duly qualified and appointed.
(c) Quorum
A majority of the Committee present in person or by telephone or other telecommunication device that permits all persons participating in the meeting to speak to each other shall constitute a quorum.
(d) Review of Charter
The Committee shall review and reassess the adequacy of this Charter at least annually and otherwise as it deems appropriate, and recommend changes to the Board of Directors. The Committee shall reference this Charter in establishing its annual goals and meeting objectives.
(e) Delegation
The Committee may delegate from time to time to any person or committee of persons any of the Committee's responsibilities that lawfully may be delegated.
(f) Reporting to the Board
The Committee will report through the Chair to the Board on matters considered by the Committee, its recommendations and performance relative to annual objectives and its Charter.
(g) Chair
The Chair shall be appointed by the Board from among the members of the Committee, and if not appointed by the Board, then shall be appointed by the members of the Committee.
(h) Absence of Chair
- 5 -
If the Chair is not present at any meeting of the Committee, one of the other members of the Committee present at the meeting shall be chosen by the Committee to preside at the meeting.
(i) Calling of Meetings
Any Director, the Chairman of the Board, the Corporate Secretary of the Company or the independent auditor of the Company may call a meeting. The Committee shall meet at least four times per year and as many additional times as needed to carry out its duties effectively.
(j) Notice of Meetings
Notice of the time and place of every meeting shall be given in writing or electronic communication to each member of the Committee at least 48 hours prior to the time fixed for such meeting. Notice of each meeting shall also be given to the independent auditors of the Company. A member of the Committee and the independent auditors may in any manner waive notice of a Committee meeting. Attendance of a member of the Committee at a meeting is a waiver of notice of the meeting except where a member attends a meeting for the express purpose of objecting to the transaction of any business on the grounds that the meeting was not lawfully called.
(k) Procedure, Records and Reporting
Subject to any statute or articles or by-laws of the Company, the Committee shall fix its own procedures at meetings, keep records of its proceedings and report to the Board, generally not later than the next scheduled meeting of the Board that follows the Committee meeting. In discharging its responsibilities, the Committee shall have full access to any relevant records of the Company.
(l) Attendance of Others at Meetings
The Committee shall have the right to determine who shall, and who shall not, be present at any time during a meeting of the Committee. The Committee may request any officer or employee of the Company, the Company's legal counsel, or any external auditor, to attend a meeting of the Committee or to meet with any members of, or consultants to the Committee. The Committee shall also have the authority to communicate directly with the independent auditor.
(m) Outside Experts and Advisors
The Committee may retain, and set and pay the compensation to, any outside expert or advisor, including but not limited to, legal, accounting, financial or other consultants, at the Company's expense, as it determines necessary to carry out its duties. The Committee will assure itself as to the independence of any outside expert or advisor.
CURRENCY OF THIS CHARTER
This Charter was last approved by the Board on November 26, 2020.