

IM Cannabis Corp.
Management's Discussion and Analysis
For the Year and Three Months Ended December 31, 2020
April 23, 2021
|
IM Cannabis Corp. |
||
|
Management’s Discussion and Analysis |
IM Cannabis Corp.
Management's Discussion and Analysis
For the Year and Three Months Ended December 31, 2020 and 2019
This Management's Discussion and Analysis ("MD&A") reports on the consolidated financial condition and operating results of IM Cannabis Corp. (the "Company" or "IMCC") for the year and three months ended December 31, 2020 and 2019. Throughout this MD&A, unless otherwise specified, references to "we", "us", "our" or similar terms, as well as the "Company" and "IMCC" refer to IM Cannabis Corp., together with its subsidiaries, on a consolidated basis, and the "Group" refers to the Company, its subsidiaries and Focus Medical Herbs Ltd.
This MD&A should be read in conjunction with the audited consolidated financial statements of the Company and notes thereto for the year ended December 31, 2020 (the "Annual Financial Statements").
The Annual Financial Statements have been prepared by management in accordance with the International Financial Reporting Standards as issued by the International Accounting Standards Board ("IFRS"). IFRS requires management to make certain judgments, estimates and assumptions that affect the reported amount of assets and liabilities at the date of the financial statements and the amount of revenue and expenses incurred during the reporting period. The results of operations for the periods reflected herein are not necessarily indicative of results that may be expected for future periods.
The Annual Financial Statements include the accounts of the Company, and the following entities:
|
Legal Entity: |
Relationship with the Company: |
|
IMC Holdings Ltd. ("IMC Holdings") |
Wholly-owned subsidiary |
|
Adjupharm GmbH ("Adjupharm") |
Subsidiary of IMC Holdings |
|
IMC Ventures Ltd. |
Subsidiary of IMC Holdings |
|
I.M.C Farms Israel Ltd. |
Wholly-owned subsidiary of IMC Holdings |
|
I.M.C. - International Medical Cannabis Portugal Unipessoal, Lda. |
Wholly-owned subsidiary of IMC Holdings |
|
Focus Medical Herbs Ltd. ("Focus") |
Private company over which IMC Holdings exercises "de facto control" under IFRS 10, as further described under the Risk Factors section below |
All intercompany balances and transactions were eliminated on consolidation.
All amounts in this MD&A are expressed in Canadian Dollars ($) in thousands, unless otherwise noted.
|
IM Cannabis Corp. |
||
|
Management’s Discussion and Analysis |
CAUTION CONCERNING FORWARD-LOOKING STATEMENTS
Certain statements in this MD&A may contain "forward-looking statements" or "forward-looking information," within the meaning of applicable securities legislation (collectively referred to herein as "forward-looking statements" or "forward-looking information"). All statements other than statements of fact may be deemed to be forward-looking statements, including statements with regard to expected financial performance, strategy and business conditions. The words "believe", "plan", "intend", "estimate", "expect", "anticipate", "continue", or "potential", and similar expressions, as well as future or conditional verbs such as "will", "should", "would", and "could" often identify forward-looking statements. These statements reflect management's current expectations and plans with respect to future events and are based on information currently available to management including based on reasonable assumptions, estimates, internal and external analysis and opinions of management considering its experience, perception of trends, current conditions and expected developments as well as other factors that management believes to be relevant as at the date such statements are made.
Without limitation, this MD&A contains forward-looking statements pertaining to:
With respect to the forward looking-statements contained in this MD&A, the Company has made assumptions regarding, among other things:
Readers are cautioned that the above lists of forward-looking statements and assumptions are not exhaustive. Since forward-looking statements address future events and conditions, by their very nature they involve inherent risks and uncertainties. Actual results may differ materially from those currently anticipated or implied by such forward-looking statements due to a number of factors and risks. These include:
|
IM Cannabis Corp. |
||
|
Management’s Discussion and Analysis |
|
IM Cannabis Corp. |
||
|
Management’s Discussion and Analysis |
Readers are cautioned that the foregoing list of risk factors is not exhaustive. Additional information on these and other factors that could affect the business, operations or financial results of the Company are detailed under the headings "Risks Factors" and "Contingent Liabilities and Commitments" of this MD&A. The Company and management caution readers not to place undue reliance on any forward-looking statements, which speak only as of the date made. Although the Company believes that the expectations reflected in the forward-looking statements are reasonable, it can give no assurance that such expectations will prove to have been correct. The Company and management assume no obligation to update or revise them to reflect new events or circumstances except as required by applicable securities laws.
FINANCIAL OUTLOOK
The forward-looking information in this MD&A contain statements in respect of estimated revenues. The Company and its management believe that the estimated revenues are reasonable as of the date hereof and are based on management's current views, strategies, expectations, assumptions and forecasts, and have been calculated using accounting policies that are generally consistent with the Company's current accounting policies. These estimates are considered financial outlooks under applicable securities legislation. These estimates and any other financial outlooks or future-oriented financial information included herein have been approved by management of the Company as of the date hereof. Such financial outlooks or future-oriented financial information are provided for the purposes of presenting information about management's current expectations and goals relating to the sales agreements described in the "Corporate Developments" section of this MD&A and other previously announced Focus sales agreements and the future business of the Company. The Company disclaims any intention or obligation to update or revise any future-oriented financial information, whether as a result of new information, future events or otherwise, except as required by securities legislation. Readers are cautioned that actual results may vary materially as a result of a number of risks, uncertainties, and other factors, many of which are beyond the Group's control. See the risks and uncertainties discussed in the "Risk Factors" section and elsewhere in this MD&A and other risks detailed from time to time in the publicly filed disclosure documents of the Company which can be viewed online under the Company's profile on the System for Electronic Document Analysis and Retrieval ("SEDAR") at www.sedar.com.
NON-IFRS FINANCIAL MEASURES
Certain financial measures used in this MD&A do not have any standardized meaning under IFRS, including "Gross Margin", "EBITDA" and "Adjusted EBITDA". For a reconciliation of these non-IFRS financial measures to the most comparable IFRS financial measures, as applicable, see the "Metrics and Non-IFRS Financial Measures" section of the MD&A.
|
IM Cannabis Corp. |
||
|
Management’s Discussion and Analysis |
OVERVIEW OF THE COMPANY
Company Background
The Company was incorporated pursuant to the Business Corporations Act (British Columbia) on March 7, 1980, under the name "Nirvana Oil & Gas Ltd." On July 12, 2013, in connection with a share consolidation, the Company changed its name to "Navasota Resources Inc.". On June 22, 2018, the Company completed a consolidation of its common shares ("Common Shares") on the basis of one post-consolidation Common Share for every five pre-consolidation Common Shares. On October 4, 2019, in connection with the Reverse Takeover Transaction (as defined below), the Company effected a consolidation of its Common Shares on the basis of one (1) post-consolidation Common Share for every 2.83 pre-consolidation Common Shares and changed its name to "IM Cannabis Corp." The Company historically engaged in mineral resource exploration activities but ceased operations in March 2018 to focus on identifying and evaluating new business opportunities. On October 11, 2019, the Company completed a business combination with IMC Holdings resulting in a reverse takeover of the Company by shareholders of IMC Holdings (the "Reverse Takeover Transaction"). The Reverse Takeover Transaction was effected by way of a "triangular merger" between the Company, IMC Holdings and a wholly-owned subsidiary of the Company pursuant to Israeli statutory law. As a result of the Reverse Takeover Transaction, the Company changed its business from mining to the international medical cannabis industry.
IMCC is a multi-country operator in the medical and recreational cannabis sector headquartered in Israel with operations in Israel, Germany and Canada.
In Israel, IMC Holdings built the IMC brand of premium medical cannabis products which have been cultivated over the last decade by Focus, an Israeli licensed cultivator over which IMC Holdings exercises "de facto control" under IFRS 10, and its cultivation partners, and sold by Focus in the Israeli market.
Focus holds a license from the MOH to propagate and cultivate medical cannabis in the State of Israel, valid until January 3, 2022 (the "Focus License"). Focus is one of the eight medical cannabis producers initially licensed by Israeli regulatory authorities and has over 10 years of experience in growing high quality medical cannabis products for the Israeli market.
As part of its core Israeli business, the Company offers intellectual property-related services to the medical cannabis industry based on proprietary processes and technologies it developed for the production of medical cannabis products. The Company offers its intellectual property and consulting services to Focus pursuant to certain commercial agreements and receives as consideration for such services a share of Focus' revenues resulting from the sale of medical cannabis products under the IMC brand.
In Europe, IMCC operates through Adjupharm, a German-based subsidiary acquired by IMC Holdings on March 15, 2019, and an EU-GMP certified medical cannabis producer and distributor with wholesale, narcotics handling, manufacturing, procurement, storage and distribution licenses granted by German regulatory authorities that allow for import/export capability with requisite permits (the "Adjupharm Licenses"). Adjupharm serves as the Company's flagship European outpost for sales and distribution.
Adjupharm currently manufactures and distributes IMC-branded medical cannabis products, in addition to other branded medical cannabis products, to pharmacies and distribution partners in Germany pursuant to sales and distribution agreements. Similar to Focus, Adjupharm sources its medical cannabis products from strategic partners, including various pan-European EU-GMP suppliers. While the Company does not currently distribute products in other European countries other than in Germany, the Company intends to leverage the platform established by Adjupharm in Germany and its network of distribution partners to expand to other jurisdictions across the continent in which medical cannabis is legal.
|
IM Cannabis Corp. |
||
|
Management’s Discussion and Analysis |
In Canada, since March 18, 2021 IMCC operates through Trichome, a Canadian-based subsidiary, and Trichome JWC Acquisition Corp. ("TJAC") d/b/a JWC, a wholly-owned subsidiary of Trichome and Canadian federally licensed producer of cannabis products in the adult-use recreational cannabis market in Canada.
IMCC is focused on further implementing an aggressive and accretive acquisition strategy focusing on attractively valued and highly synergistic targets in Canada. The consolidated revenues of the Group for the twelve months ended December 31, 2020, was generated mainly from the sale of medical cannabis products in Israel and Germany, by Focus and Adjupharm, respectively. The Group does not engage in any U.S. cannabis-related activities as defined in Canadian Securities Administrators Staff Notice 51-352 (Revised) - Issuers with U.S. Marijuana-Related Activities.
As of December 31, 2020, the Company's major Israeli assets include the Commercial Agreements and the Focus Agreement, as well as holdings in Xinteza API Ltd. ("Xinteza").
As of December 31, 2020, the Company's major international assets include material holdings in Adjupharm, a fully licensed medical cannabis distribution company in Germany and a 25% interest in a cultivation joint venture in Greece.
Neither the Company nor any of its subsidiaries currently hold, directly or indirectly, any licenses to engage in the propagation, cultivation, production, processing, distribution or sale of medical cannabis products in Israel as required by local legislation. However, under IFRS 10, the Company is required to consolidate the results of Focus, a licensed propagator and cultivator of medical cannabis products under the current Israeli regulatory regime. Focus operates under the regulations of medical cannabis products by the MOH through the IMCA to propagate and cultivate medical cannabis products in Israel. As such, all financial information in this MD&A is presented on a consolidated basis reflecting the results of the Group. All of Focus' operations are performed pursuant to the Dangerous Drugs Ordinance and the related regulations issued by IMCA. While IMCC does not hold any of the Israeli licenses mentioned above and does not own Focus, it derives a significant portion of its consolidated revenues from Focus' revenue, which is primarily earned from the medical cannabis sales agreements that Focus has with various pharmacies in Israel. Furthermore, the Company has an option under the Focus Agreement to re-acquire 74% ownership of Focus. For more information, please see at the Risk Factors section below.
Company Products
'IMC' is a well-known medical cannabis brand in Israel. Leveraging its long-term success in the Israeli market, the Company launched the brand in Germany in 2020. The Company believes that the IMC brand in Israel has become synonymous with quality and consistency in the Israeli medical cannabis market and it was chosen as one of the four top favourite brands in Israel.1
_________________________________
1 According to a survey carried out by Cannabis Magazine among 519 patients licensed by the MOH to consume medical cannabis (Aug2020, Israel).
|
IM Cannabis Corp. |
||
|
Management’s Discussion and Analysis |
In association with Focus, the Company maintains a brand portfolio that includes popular medical cannabis inflorescences such as Roma, Dairy Queen, London, Tel Aviv and Pandora Box, as well as full-spectrum cannabis extracts.
'Roma' is marketed as an elegant strain that is known for its strong impact and influence. Roma was chosen as one of the most favored strains in Israel.2 'Tel-Aviv' is marketed as sativa dominated strain that is known for uplifting the spirit and enhancing creativity. Both Roma and Tel-Aviv contain THC, CBD, and CBN within the following ranges: 16-24% (THC), 0-7% (CBD), and CBN lower than 1.5%.
'London' is marketed as a distinct indica, which stands out due to its flavor and strong influence. 'Dairy Queen' is marketed as a rich, velvety strain with a cherry aroma that may assist with reducing stress and producing calmness. 'Pandora Box' is marketed as a sativa dominate strain, which confers a sense of spirit uplifting, energy and vitality. London, Dairy Queen, and Pandora Box contain THC, CBD, and CBN within the following ranges: 11-19% (THC), 0-5.5% (CBD), and CBN lower than 1.5%.
All of the products are tested in certified labs according to MOH standards and certified before being packaged and labelled with detailed information about the THC, CBD and CBN content of each product.3
In Germany, the Company sells an IMC-branded medical cannabis inflorescence product. The medical cannabis product sold in the German market is branded generically as "IMC" so as to rely on the Company's brand recognition in establishing a foothold with German healthcare professionals.
In Canada, commencing March 18, 2021, following completion of the Trichome Transaction (as defined below), the Company's product portfolio consists of primarily dried inflorescence, pre-rolled cannabis, pressed hash and kief offerings sold by TJAC under the JWC Brand into the Canadian adult use recreational cannabis market. Dried inflorescence is sold primarily in 3.5 gram, 14 gram and 28 gram formats, all pre-rolls were sold in a 3 x 0.5 gram format and both hash and kief sold in 1 gram and 2 gram formats.
In 2021, TJAC will continue to offer its existing product portfolio and plans to introduce additional offerings in the form of new dried inflorescence strains, new packaging formats and a rebranding of its dried inflorescence, pre-rolled cannabis, hash and kief products under the company's recently launched Wagners brand. The Company is focused on diversifying its product portfolio, mainly with premium and super premium branded cannabis products both is Israel, in association with Focus, and in the European market through Adjupharm.
_________________________________
2 According to a survey carried out by Cannabis Magazine among 519 patients licensed by the MOH to consume medical cannabis (Aug2020, Israel).
3 The actual percentages of THC and CBD content are determined by certified laboratory inspections and disclosed on the label of each IMC-branded medical cannabis product sold in Israel. Depending on such THC and CBD content, each IMC-branded medical cannabis product is labelled based on the following categories, in accordance with MOH Regulations: (a) 'T20/C4' (THC 16-24% and CBD 0-7%); (b) 'T15/C3' (THC 11-19% and CBD 0-5.5%); (c) 'T10/C2' (THC 6-14% and CBD 0-3.8%); (d) 'T10/C10' (THC 6-14% and CBD 6-14%); (e) 'T5/C5' (THC 1-9% and CBD 1-9%); (f) 'T0/C24' (THC 0-0.5% and CBD 20-28%); (g) 'T1/C20' (THC 0-2.5% and CBD 16-24%); (h) 'T3/C15' (THC 0.5-5.5% and CBD 11-19%); and (i) 'T5/C10' (THC 2.5-7.5% and CBD 6-14%). The stated THC, CBD and CBN percentage ranges for the IMC branded strains are expected ranges; the actual percentages, as labelled on product packaging under the IMC brand, may vary or deviate from such ranges.
|
IM Cannabis Corp. |
||
|
Management’s Discussion and Analysis |
Corporate Developments
(i) Corporate Restructuring and Canadian Liquidity Events
In June 2018, the Company announced the entering into a letter of intent with IMC Holdings pursuant to which IMC Holdings would complete a reverse takeover of the Company and a change of business of the Company from mining to the medical cannabis industry (the "Reverse Takeover Transaction"). In November 2018, the Company and IMC Holdings announced the entering into of a definitive business combination agreement whereby the reverse takeover would be completed by way of a three-cornered amalgamation involving the parties and a wholly-owned subsidiary of the Company, Navasota Acquisition Ltd. ("Navasota Subco"). On September 3, 2019, IMC Holdings, Navasota and Navasota Subco amended and restated the business combination agreement which superseded the previous agreement signed in November 2018.
On August 30, 2019, Navasota and IMC Holdings announced the completion of a private placement offering of 19,460 , 527 subscription receipts (each a "Subscription Receipt") of a wholly-owned subsidiary of Navasota ("Finco") at a price of $1.05 per Subscription Receipt for aggregate gross proceeds of $20,43 3 (the "Financing") pursuant to the terms of the Reverse Takeover Transaction. Upon the satisfaction or waiver of, among other things, all of the condition precedents to the completion of the Reverse Takeover Transaction, each Subscription Receipt was exchanged for one unit of Finco (a "Finco Unit") with each Finco Unit being comprised of one (1) common share of Finco (a "Finco Share") and one-half (1/2) of one (1) common share purchase warrant of Finco (a "Finco Warrant"). Each whole Finco Warrant was exercisable for one Finco Share at an exercise price of $1.30 for a period of 24 months following the closing of the Reverse Takeover Transaction. Upon closing of the Reverse Takeover Transaction, the Finco Shares and Finco Warrants were exchanged for Common Shares and IMCC warrants ("Warrants") on economically equivalent terms on a 1:1 basis.
The Warrants included in each 2019 Compensation Option were determined to be a financial derivative and accordingly were classified as financial liability measured at fair value through profit or loss. Accordingly, the Company allocated the gross proceeds received to the securities issued in the 2019 Compensation Options, such that proceeds allocated to the Warrants component based on their fair value on the date of the placements amounted to $2,597 and proceeds allocated to the Common Shares were determined to be the residual amount of $17,836.
In addition, IMC Holdings granted to the Agents options to acquire 1,199,326 compensation units (the "2019 Compensation Units") at an exercise price of $1.05 per 2019 Compensation Unit. Upon the Reverse Takeover Transaction, the Compensation Units were exchanged for compensation options of the Company (the "2019 Compensation Options"). Prior to the Share Consolidation, each 2019 Compensation Unit consisted of one Common Share and one half Warrant with each whole Warrant exercisable for one Common Share at an exercise price of $1.30 for 36 months following the issuance.
Issuance expenses in the amount of $3,337 (including the fair value of the 2019 Compensation Options amounting to $741) were allocated as follows: $424 to the Warrants was expensed in finance expense in the consolidated statement of profit or loss and other comprehensive income and $2,913 was allocated to the Common Shares and recorded as a reduction of share premium.
|
IM Cannabis Corp. |
||
|
Management’s Discussion and Analysis |
Pursuant to the terms of the Reverse Takeover Transaction, on October 4, 2019, Navasota completed a consolidation of its common shares on 2.83:1 basis and changed its name to "IM Cannabis Corp.". On October 11, 2019, the Reverse Takeover Transaction was completed, which included the merger of IMC Holdings and Navasota Subco under Israeli laws and the resulting amalgamated entity becoming a wholly-owned subsidiary of IMCC. Upon the completion of the Reverse Takeover Transaction, the former holders of IMC Holdings ordinary shares (the "IMC Ordinary Shares") held approximately 84.28% of the issued and outstanding Common Shares, the previous holders of Subscription Receipts held approximately 13.35% of the Common Shares and the previous holders of Navasota shares held 2.37% of the Common Shares, in each case, on a non-diluted basis.
On November 5, 2019, the Common Shares began trading on the CSE under the ticker symbol "IMCC".
In June 2020, the Company received $6,032 proceeds from the exercise of Warrants (the "2018 Warrants") and compensation options of the Company (the "2018 Compensation Options" and together with the 2018 Warrants, the "2018 Warrants and Compensation Options"), which were issued in May through June, 2018, with expiration dates between May through June, 2020. A total of 12,350,795 of the 2018 Warrants and Compensation Options were exercised, representing 92.1% of the total quantity of the 2018 Warrants and Compensation Options, at a price of $0.50 per Warrant and $0.40 per 2018 Compensation Option. The 2018 Warrants, which were accounted for as a liability, were revalued to their fair value immediately prior to their exercise. A revaluation in the amount of $3,675 was recorded as finance expenses. The carrying amount of the liability was reclassified to equity upon the exercises of the 2018 Warrants. The unexercised 2018 Warrants and Compensation Options have since expired.
On February 12, 2021, the Company's shareholders approved at a special meeting the consolidation of all the Company's issued and outstanding Common Shares on a four (4) to one (1) basis (the "Share Consolidation"). Following the Share Consolidation, the number of Warrants outstanding was not altered; however, the exercise terms were adjusted such that four Warrants are exercisable for one Common Share following the payment of an adjusted exercise price of $5.20. The consolidated financial statements give effect to the Share Consolidation for all periods presented.
On March 1, 2021, the Company's Common Shares commenced trading on NASDAQ capital market ("NASDAQ") under the ticker symbol "IMCC", making the Company the first Israeli medical cannabis operator to list its shares on NASDAQ.
As of December 31, 2020 and 2019, there were nil and 11,413,750 (after effect of split 1:10 in IMC Holdings) 2018 Warrants outstanding, respectively, with fair value in the amount of $nil and $197, respectively. For the years ended December 31, 2020 and 2019, the Company recognized a revaluation loss (gain) of $3,675 and ($856), respectively, in the consolidated statement of profit or loss and other comprehensive income, in which the unrealized gain is included in finance income (expense).
As of December 31, 2020 and 2019, there were 9,729,264 and 9,730,264, respectively, Warrants outstanding issued in connection with the 2019 Private Placements, respectively, and the Company re-measured the Warrants, according to their trading price in the market, in the amount of $16,540 and $nil, respectively. As a result, for the years ended December 31, 2020 and 2019, the Company recognized a revaluation loss (gain) of $16,283 and ($2,597), respectively, in the consolidated statement of profit or loss and other comprehensive income, in which the unrealized gain is included in finance income (expense).
|
IM Cannabis Corp. |
||
|
Management’s Discussion and Analysis |
In addition, as of December 31, 2020 and 2019, there were nil and 706,713 Warrants outstanding, respectively, from the issuance to certain Navasota shareholders in the Reverse Takeover Transaction. During 2020, a total of 113,520 Warrants were exercised for Common Shares at an exercise price of $0.283, and the remaining Warrants issued to Navasota shareholders expired on April 13, 2020.
During the year ended December 31, 2020, a total of 1,000 Warrants issued in connection with the 2019 Private Placements were exercised at an exercise price of $1.30 per Warrant. As a result, the Company received a total amount of $1, at a price of $1.30 per 2019 Warrant.
During the year ended December 31, 2020, a total of 327,780 Compensation Options were converted to 327,780 Common Shares and 163,890 Warrants. Consequently, the Company received a total amount of $344.
(ii) Restructuring
Current Israeli law requires the prior approval by the IMCA of the identity of any shareholder owning 5% or more of an Israeli company licensed to engage in cannabis-related activities. For a number of reasons, including the opportunity to leverage a network of multiple Israeli licensed producers cultivating under the IMC brand, and in contemplation of a "go-public transaction" to geographically diversify the Company's share ownership, IMC Holdings restructured its organization on April 2, 2019 (the "IMC Restructuring") resulting in the divestiture to Oren Shuster and Rafael Gabay of its interest in Focus, which is licensed by the MOH to propagate and cultivate cannabis in Israel.
IMC Holdings retains an option with Messrs. Shuster and Gabay to re-acquire the sold interest in Focus at its sole discretion and in accordance with Israeli cannabis regulations, within 10 years of the date of the IMC Restructuring (the "Focus Agreement"). The Focus Agreement sets an aggregate exercise price equal to NIS 765.67 per share of Focus for a total consideration of NIS 2,756,500, that being equal to the price paid by Messrs. Shuster and Gabay for the acquired interests in Focus at the time of the IMC Restructuring.
As part of the IMC Restructuring, IMC Holdings and Focus entered into an agreement in which Focus shall use the IMC brand on an exclusive basis for the sale of any cannabis plant and/or cannabis product produced by Focus, either alone or together with other sub-contractors engaged by Focus (the "IP Agreement"). Focus is also obligated to exclusively use IMC Holdings for certain management and consulting services including: (a) business development services; (b) marketing services; (c) strategic advisory services; (d) locating potential collaborations on a worldwide basis; and (e) financial analysis services (the "Services Agreement" and collectively with the IP Agreement, the "Commercial Agreements").
Under the IP Agreement, IMC Holdings charges Focus an amount equal to 25% of its revenues on a quarterly basis, which shall not be changed without the consent of IMC Holdings, as consideration for Focus' use of certain trademarks, know-how, technology and maintenance services provided by IMC Holdings.
Under the Services Agreement, IMC Holdings charges Focus an amount equal to IMC Holdings' cost of providing certain services to Focus plus a 25% mark-up, which shall not be changed without the consent of IMC Holdings, as consideration for the provision of such services.
Subsequent to the IMC Restructuring, according to accounting criteria in IFRS 10, IMCC is still viewed as effectively exercising control over Focus, and therefore, the accounts of Focus continue to be consolidated with those of the Company.
|
IM Cannabis Corp. |
||
|
Management’s Discussion and Analysis |
As a result of the IMC Restructuring, IMCC derives revenue from the Commercial Agreements. IMCC does not directly hold any licenses to engage in the cultivation, production, processing, distribution or sale of medical cannabis in Israel.
(iii) Regulatory Changes in Israel
Changes under the MOH Regulations
Until September 2019, patients licensed for consumption of medical cannabis products by the IMCA received all of their medical cannabis products authorized under their respective licenses at a fixed monthly price of NIS 370, regardless of each patient's authorized amount. As an example, a patient who was to receive 20 grams of medical cannabis products per month would pay the same monthly fee of NIS 370 as a patient who received 180 grams per month. In addition, IMCA assigned patients to a particular licensed medical cannabis producer, from which each patient would exclusively receive their medical cannabis products. Under the previous medical cannabis regulations, Focus distributed approximately 80% of its medical cannabis products via home delivery and the remaining 20% via an IMCA-established distribution outlet.
Under the MOH's new regulations, medical cannabis products are delivered from a licensed producer to a manufacturer, which then delivers to a distributor to distribute to pharmacies. In addition, patients licensed for consumption of medical cannabis products are no longer exclusively assigned to medical cannabis producers and may purchase medical cannabis products from authorized pharmacies at a range of price points without any MOH-regulated price controls.
In light of the MOH's new regulations, some medical cannabis patient licenses granted under the previous regime are still valid. The medical cannabis patient licenses set to expire during the period from February 1, 2019 to July 31, 2019 were extended by order of the Israeli Supreme Court until further notice by the Court. While these licenses remain valid, the patients who hold these licenses are entitled to receive medical cannabis products pursuant to the price controls and supplier restrictions of the former regime. Additional information on the proceedings pursuant to which the above-referenced order was granted can be found under "Legal Proceedings and Regulatory Actions - Legal Proceedings - Supreme Court of Justice 2335/19".
Following the implementation of the above MOH's new regulations, the Group believes that the Israeli medical cannabis market will continue to benefit from price stability of the premium and super premium medical cannabis products, an increase to the number of physicians certified by the IMCA to prescribe medical cannabis and thus, an increase in the number of licensed medical cannabis patients.
Medical Cannabis Imports
In October 2020, the MOH issued an updated procedure, titled "Guidelines for Approval of Applications for Importation of Dangerous Drug of Cannabis Type for Medical Use and for Research" ("Procedure 109"), describing the application requirements for cannabis import licenses for medical and research purposes. According to Procedure 109, the following permits and licenses are required to receive a cannabis import license: (1) License to possess medical cannabis and operate in the medical cannabis industry; (2) License to import plant material; (3) Permit to import narcotic drugs; and (4) License to import a dangerous drug.
|
IM Cannabis Corp. |
||
|
Management’s Discussion and Analysis |
Medical Cannabis Exports
In October 2020, the MOH launched a new pilot program under which medical cannabis producers would be authorized to export medical cannabis products, subject to the requirement that certain products be made available at a fixed price of NIS 14 per gram to patients in Israel over the age of 21 and NIS 10 per gram to patients under the age of 21 (the "Pilot Program"). Each participating company would decide the selection of medical cannabis products made available under the Pilot Program. The Pilot Program was planned for an initial period of three months and was extended in January 2021. As products bearing the IMC brand are offered as part of the Pilot Program, IMC-branded products are eligible for immediate application for export permits.
In December 2020, the IMCA published guidelines for the medical cannabis export permit application process4 (the "Export Guidelines"), pursuant to which an export permit will only be granted to an applicant if (i) sufficient domestic supply has been secured by such applicant in the variety and quantity that will meet the Israeli level of demand; (ii) the delivery of medical cannabis is made from approved sites; (iii) the applicant has a valid IMC-GDP certification and business license from the IMCA; and (iv) an import permit from the importing country is obtained and attached to the export application. The term to apply for export permits under the program, according to the Export Guidelines, were set to expire at the end of Q1 2021. Further extensions are considered by the IMCA based on the success of the Pilot Program.
Legalization of Adult-Use Recreational Cannabis in Israel
As of the date of this MD&A, adult-use recreational cannabis use in Israel is illegal. In November 2020, an Israeli government committee responsible for advancing the cannabis market reform published a report supporting and recommending the legalization of adult-use recreational cannabis in Israel (the "Report"). Based on the Report, the Israeli Ministry of Justice was expected to formulate a bill to begin the legislative process towards the legalization of adult-use recreational cannabis. The government committee made its recommendation for legalization based on the increasing demand for adult-use recreational cannabis in Israel, the importance of maintaining quality standards and limiting uncontrolled products, the need for increased access to cannabis by medical patients and the objective of decreasing the size of the illegal market. The model proposed by the government committee in the Report is similar in nature to the model adopted in Canada, whereby the sale of adult-use recreational cannabis would be channeled through government-licensed dispensaries.
In December 2020, the governing Israeli parliament dissolved and general elections were scheduled for March 2021. All such legislative initiatives were suspended and there is no certainty regarding their renewal following a formation of a new government pursuant to the March 2021 elections.
(iv) Israeli Market
The Israeli medical cannabis market has shown dramatic growth over the past several years. It is projected that this growth will continue and according to MOH estimates, the number of patients in Israel licensed by the MOH to consume medical cannabis is expected to reach 120,000by the end of 2021.
Israeli Market Development 2011-2021
_________________________________
4 Directive 110, December 2020 [Hebrew] - https://www.health.gov.il/hozer/CN_110.pdf
|
IM Cannabis Corp. |
||
|
Management’s Discussion and Analysis |
According to MOH monthly publication, as of March 2021, there are 88,428 licensed patients in Israel, and a monthly prescription of 2,848,000 and 3,190,000 grams of cannabis were recorded in December 2020 and March 2021, respectively.5
The below reflects the number of licensed medical cannabis patients in Israel over the year 2011 to March 2021:
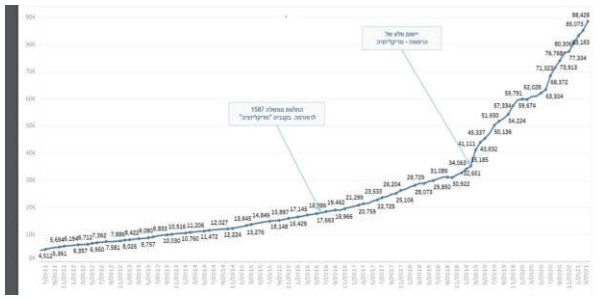
(v) European Activity
The Company's European strategy is centered in Germany, whose medical cannabis market is currently considered the largest in Europe.6 To develop its operations in Germany, on March 15, 2019, the Company acquired, through IMC Holdings, 100% of the shares of Adjupharm (the "Adjupharm Shares"), a licensed EU-GMP certified medical cannabis distributor. IMC Holdings acquired the Adjupharm shares for €924 (approximately $1,400) with additional obligations to the sellers including repayment of bank loans of up to €680 (approximately $1,030). These bank loans were repaid by IMC Holdings in May 2019. The Company, through IMC Holdings, currently owns 92.5% of Adjupharm, with the balance owned by Adjupharm's Chief Executive Officer. An additional 2.48% ownership stake in Adjupharm will be granted to Adjupharm's Chief Executive Officer once entitled, pursuant to the terms of his employment agreement.
The Company continues to develop Adjupharm as its European hub and to expand its presence in the German market by forging partnerships with pharmacies and distributors across the country. Led by Adjupharm's Chief Executive Officer MR. Richard Balla, the Company's objective is to capture a significant market share in Germany by working directly with distributors to increase market reach for products bearing the IMC brand. The Company currently has approximately 3,200 square feet of warehousing and GMP Standard production capacity in Germany and is in process to expand its facilities by an additional 3,200 square feet. Adjupharm sources its supply of medical cannabis for the German market from EU-GMP certified suppliers.
_________________________________
5 https://www.health.gov.il/Subjects/cannabis/Documents/licenses-status-march-2021.pdf
6 Health Europa, June 23, 2020. https://www.healtheuropa.eu/exploring-growth-in-the-european-medical-cannabis-market/100849/
|
IM Cannabis Corp. |
||
|
Management’s Discussion and Analysis |
Adjupharm relies on its sales and distribution agreements to supply and distribute IMC-branded products to distribution partners in Germany, which are then distributed to German pharmacies. There are approximately 19,000 community pharmacies in Germany, each of which is permitted to create and dispense medications, including medical cannabis, pursuant to physician prescriptions.7 Adjupharm recently completed the expansion of its internal and external sales department and is focused on increasing physician awareness and engagement to drive sales of IMC-branded medical cannabis products. The competitive advantage in Germany lies in the Group's track record and brand reputation in Israel and proprietary data supporting the effectiveness of medical cannabis for the treatment of a variety of conditions.
The Company is actively seeking additional cultivation partners to diversify its sources of supply of premium and super premium cannabis products and further develop its European presence.
The Company has also engaged in exploratory operations to expand to Portugal and Greece, by establishing a wholly-owned subsidiary in Portugal in October 2018, and a joint venture in Greece (25% owned by IMCC), however it has deferred any further investment in these jurisdictions indefinitely in light of the uncertainty related to COVID-19.
Due to the impact of the COVID-19 pandemic on Germany in the first quarter of 2021, the Company, through Adjupharm, leveraged its established distribution platform to enter into several reseller agreements of COVID-19 antigen test kits. In light of the uncertainty related to COVID-19, the Company will examine the continued demand of the German market for such test kits prior to any further engagement relating thereto. For more information, please see "Subsequent Events".
(vi) Investment in Xinteza
On December 26, 2019, IMC Holdings entered into a share purchase agreement with Xinteza, a company with a unique biosynthesis technology, whereby the Company acquired, on an as-converted and fully diluted basis, 25.37% of Xinteza's outstanding share capital, for consideration of US$1,700 (approximately $2,223, according to the December 24, 2019 exchange rate published by the Bank of Canada) paid in several installments (the "Xinteza SPA"). As of December 31, 2020, the Company has paid all outstanding installments pertaining to the Xinteza SPA and holds 24.2% of the outstanding share capital of Xinteza on an as-converted and fully diluted basis.
Under an exclusive license from Yeda Research & Development Company Ltd., the commercial division of the Weizmann Institute of Science, and based on disruptive plant genetics and metabolomics research led by Professor Asaph Aharoni, Xinteza has been developing advanced proprietary technologies relating to the production of cannabinoid-based active pharmaceutical ingredients for the pharmaceutical and food industries using biosynthesis and bio-extraction technologies.
_________________________________
7 Federal Union of German Associations of Pharmacists: Figures Data Facts 2020.
|
IM Cannabis Corp. |
||
|
Management’s Discussion and Analysis |
(vii) Strategic Developments:
1. On January 23, 2020, IMC Holdings entered into definitive agreements to establish a medical cannabis cultivation and processing joint venture in Greece with Galen Industries Single Member Societe Anonyme ("Galen"), a Greek company established by a consortium of investors in Greece with extensive experience in the pharmaceutical, media, finance and energy sectors. As a result of these agreements, IMC Holdings acquired ownership of 25% of the paid-up capital of Shiran Single Member Societe Anonyme ("Shiran") a private company incorporated and registered in Greece and originally wholly-owned by Galen, while the remaining 75% remained under the ownership of Galen. Under the agreements, each party is committed to fund the initial capital expenditures, totaling approximately up to EUR 8,000,000 for the construction of an EU-GMP certified cultivation and processing facility in Greece.
Also on January 23, 2020, Shiran, Galen and IMC Holdings signed a preferred supply agreement (the "Galen Supply Agreement"). Under the Galen Supply Agreement, IMC Holdings has the right to purchase up to 25% of the total production of Shiran at a preferred price as determined therein, for an initial period of five years. As of the date of this MD&A, no material capital expenditures have been made towards Shiran given the uncertainty relating to COVID-19. The Company is deferring any further investment into Greece indefinitely.
2. On March 23, 2020, Focus signed a supply agreement (the "Intelicanna Supply Agreement") with Intelicanna Ltd. ("Intelicanna") for the purchase by Focus of a minimum of 500kg and up to 1,000kg of medical cannabis cultivated by Intelicanna. Additional purchases may be made by Focus under the Intelicanna Supply Agreement without a change to the contracted price paid to Intelicanna. The finished products are to be sold to pharmacies in Israel under the IMC brand. The Intelicanna Supply Agreement is in effect for a term of 12 months from the date of the first planting in Intelicanna's facility. Intelicanna has received access to certain Focus unique and proprietary genetics for the sole purpose of delivering product under the Intelicanna Supply Agreement; however, the genetics remain the exclusive property of Focus. Under the Intelicanna Supply Agreement, Intelicanna is responsible for all production activities under Focus' supervision and quality control practices throughout the growing process at Intelicanna's site.
3. On March 30, 2020, Focus signed a binding three-year sales agreement for the sale of IMC-branded medical cannabis products (the "March 2020 Pharmacy Sales Agreement") to three pharmacies in Jerusalem operating under the Oranim Pharm and Medi Plus banners. Pursuant to the March 2020 Pharmacy Sales Agreement, Focus is to supply such pharmacies with a total of 800kg of medical cannabis products annually for a period of three years, commencing in 2021, for an aggregate amount of 2,400kg of medical cannabis products at a contracted price.
4. On March 31, 2020, Focus signed a supply agreement with Way of Life Ltd., an IMC-GAP certified cultivator ("Way of Life"), and Cannation Ltd., an IMC-GAP applicant ("Cannation", and together with Way of Life, the "Suppliers") to purchase a total of approximately 2,600kg of medical cannabis per year for an aggregate amount of up to 7,800kg of medical cannabis products over three years. Of the aggregate amount to be supplied under the agreement, delivery of 6,200kg was contingent upon Cannation receiving its IMC-GAP certification. All finished products produced from the medical cannabis supplied under such supply agreement will be sold under the IMC brand to pharmacies in Israel. Under the supply agreement, the Suppliers obtained access to certain Focus unique and proprietary genetics for the sole purpose of cultivating and delivering medical cannabis; however, the genetics would remain the exclusive property of Focus. In addition, Focus received access to the Suppliers' growing facilities to monitor the entire growing process. As Focus has secured the necessary supply to fulfill its delivery obligations under its pharmacy sales agreements and support its Israeli operations, and following the expiration of the milestone for Cannation to obtain IMC-GAP certification, the supply agreement with Cannation was terminated on November 24, 2020.
|
IM Cannabis Corp. |
||
|
Management’s Discussion and Analysis |
5. On April 2, 2020, the Company announced that Adjupharm had received the necessary approvals from regulatory authorities to begin imports and sales of medical cannabis products under the IMC brand to German patients.
6. On April 6, 2020, Focus signed a binding two-year sales agreement for the sale of medical cannabis products under the IMC brand with Shor Tabachnik pharmacies ("Tabachnik") (the "Tabachnik Sales Agreement"). According to the Tabachnik Sales Agreement, Focus will sell to Tabachnik 1,000kg of medical cannabis products under the IMC brand annually for the duration of the Tabachnik Sales Agreement at an agreed upon price beginning in 2021.
7. On April 13, 2020, Focus signed a binding three-year agreement for the sale of 13,575kg of medical cannabis products under the IMC brand to Super-Pharm (Israel) Ltd. ("Super-Pharm"), the largest pharmacy chain in Israel (the "SP Sales Agreement"). According to the SP Sales Agreement, Focus will sell to Super-Pharm a total of 13,575kg of medical cannabis products under the IMC brand over the next three years. Medical cannabis products sold under the SP Sales Agreement will include both inflorescence and extract products at an agreed upon price.
8. On April 13, 2020, Focus signed a one-year binding agreement for the sale of 1,000kg of medical cannabis products under the IMC brand to Panaxia Labs Israel, Ltd. at an agreed upon price.
9. On April 14, 2020, Focus signed an agreement for the sale of up to 1,500kg of medical cannabis products under the IMC brand to Max Pharm Ltd. ("Max Pharm") over a three-year period (the "MP Sales Agreement"). Under the MP Sales Agreement, Focus will sell to Max Pharm a total of 500kg of medical cannabis products under the IMC brand annually at an agreed upon price beginning in 2021. Max Pharm has an option to purchase an additional 500kg of medical cannabis products from Focus in each of 2021, 2022 and 2023, for a total volume of up to 3,000kg over three years.
10. On April 21, 2020, Focus signed a binding three-year agreement for the sale of 12,600kg of medical cannabis products under the IMC brand to PharmYarok Ltd. ("PharmYarok") (the "PY Sales Agreement"). According to the PY Sales Agreement, Focus will sell to PharmYarok a total of 12,600kg of medical cannabis products under the IMC brand between 2021 and 2023 at an agreed upon price, subject to PharmYarok meeting certain regulatory requirements. Medical cannabis products sold under the PY Sales Agreement may include both inflorescence and extract products.
11. On April 26, 2020, Focus signed a three-year definitive supply agreement (the "Megadim Supply Agreement") with an IMC-GAP certified independent farmer located in Megadim, Israel and licensed to cultivate medical cannabis. Under the Megadim Supply Agreement, Focus will purchase a total of up to 8,060kg of medical cannabis over three years at an agreed upon price, of which approximately 7,500kg is contingent upon the supplier meeting quality criteria set under the Megadim Supply Agreement. All finished products created from the medical cannabis pursuant to the Megadim Supply Agreement will be sold by Focus under the IMC brand to pharmacies in Israel. On February 10, 2021, the Company announced the amendment to the Megadim Supply Agreement, to reflect the supply of only three harvests of medical cannabis being purchased by Focus. Under such amendment and subject to the terms therein, upon payment for all three harvests, the Megadim Supply Agreement will be terminated. Following this change, approximately 570 kg of medical cannabis was provided to Focus by the supplier.
|
IM Cannabis Corp. |
||
|
Management’s Discussion and Analysis |
12. On May 7, 2020, the Company announced that Adjupharm received purchase orders for an aggregate of 360kg of IMC-branded medical cannabis products pursuant to certain distribution agreements entered into with German distributors in March 2020.
13. On May 8, 2020, Adjupharm received regulatory confirmation for the import of up to 5,800kg of medical cannabis products into Germany from foreign suppliers under the Adjupharm licenses within a 12-month period. Such confirmation allows Adjupharm to import either bulk products, such as inflorescences and dronabinol, or extract products for end-products, at specified quantities set out in the confirmation.
14. On May 12, 2020, the Company announced that Adjupharm received a purchase commitment from a distributor in Germany for 465kg of IMC-branded medical cannabis products over a 12-month period.
15. On May 26, 2020, Focus received its first shipment of 200kg of imported medical cannabis from Spain-based Linneo Health S.L, the Company's EU-GMP certified supply partner for medical cannabis, which in June 2020, began selling in Israel under the IMC brand.
16. On June 12, 2020, the Company signed a binding term sheet for the exclusive distribution rights of CannEpil® in Israel for a period of five years (the "CannEpil Term Sheet"), subject to CannEpil® meeting requirements under applicable laws to be qualified as a legal drug in Israel. CannEpil® is a phytocannabinoid medicine developed by MGC Pharmaceuticals Ltd. ("MGC") for the treatment of refractory epilepsy. According to the CannEpil Term Sheet, IMCC would be responsible for the registration, promotion and distribution of CannEpil® in Israel. IMCC would also obtain all necessary permits and licenses for importation and commercialization. MGC would continue to own all intellectual property rights associated with CannEpil® and its continued research and development.
17. On June 18, 2020, Focus received its first imported shipment of medical cannabis from a Canadian EU-GMP certified medical cannabis cultivator. The shipment was comprised of approximately 200kg of medical cannabis to be sold by Focus under the IMC brand to pharmacies in Israel.
18. In July 2020, Adjupharm entered into several binding medical cannabis sales agreements with the following distributors in Germany: Zur Rose Pharma GmbH ("Zur Rose"), Axicorp Group, Canymed GmbH and Materia Deutschland GmbH. These additional distributors brought Adjupharm's total number of contracted German distributors to seven, with definitive purchase commitments with such distributors totaling 1,525kg of medical cannabis products bearing the IMC brand to be delivered in Germany over a 12-month period. A settlement to terminate the medical cannabis sales agreement with Zur Rose was reached on March 30, 2021.
On March 30, 2021, subsequent to the reporting period, Zur Rose and the Company entered into a termination settlement agreement according to Zur Rose's request, according to which, Adjupharm received a termination fee. According to the termination agreement no inventory will be transferred from Zur Rose to Adjupharm or the opposite.
19. On July 24, 2020, Focus signed a supply agreement with Ever Green Solomon Pharma Ltd ("Ever Green") (the "Ever Green Supply Agreement"), an IMC-GAP certified cultivator, for the purchase of all of the medical cannabis production cultivated by Ever Green in an 86,000 square feet area of its facility, over a period of five years, with an option for Focus to extend the term by an additional five years. The finished products created from medical cannabis delivered pursuant to the Ever Green Supply Agreement will be sold by Focus to pharmacies in Israel under the IMC brand.
|
IM Cannabis Corp. |
||
|
Management’s Discussion and Analysis |
20. On July 28, 2020, the Company established a wholly-owned subsidiary in the Netherlands, IMC Holland, which subsequently established another Dutch entity, IMC Holland B.V. ("Holland B.V."), in which 60% is owned by IMC Holland, and the remaining 40% is owned by a group of four individuals with expertise in the Dutch cannabis market. Holland B.V. was incorporated for the purpose of applying for a Dutch governmental tender (the "Dutch Tender") and to establish a full cannabis supply chain to coffee shops in the Dutch municipalities participating in the Dutch Tender. On November 27, 2020, the Company received notice that its application for the Dutch Tender was not accepted. Accordingly, Holland B.V. was liquidated effective as of December 18, 2020. As of the date of this MD&A, the Company is exploring other strategic opportunities involving successful applicants of the Dutch Tender but does not currently have any material operations in the jurisdiction.
21. On September 8, 2020, Adjupharm signed distribution agreements for the sale of IMC-branded medical cannabis products with Cansativa GmbH and Ilios Sante GmbH.
22. On September 9, 2020, Adjupharm signed a distribution agreement for the sale of IMC-branded medical cannabis products with Farmako GmbH, bringing its total number of contracted German distributors to ten.
23. On September 15, 2020, the Company imported its first shipment of medical cannabis from its EU-GMP supply partner into Germany for distribution and sale through its German distributors, under the IMC brand.
24. On September 23, 2020, the Company officially launched the IMC brand in Germany as four of the Company's German distribution partners received shipments of medical cannabis products for sale in the German medical cannabis market. The first product bearing the IMC brand available to customers was the High THC T20/1 medical cannabis inflorescence.
25. On December 30, 2020, the Company entered into a definitive agreement with Trichome, to combine their businesses pursuant to a plan of arrangement to be completed under the Business Corporations Act (Ontario) (the "Trichome Transaction").
Subsequent Events
1. On February 12, 2021, the Company's shareholders approved at a special meeting, the Share Consolidation.
2. On March 1, 2021, the Company's Common Shares commenced trading on NASDAQ under the ticker symbol "IMCC", making the Company the first Israeli medical cannabis operator to list its shares on NASDAQ.
3. On March 8, 2021, the Company announced that Focus signed a multi-year supply agreement with GTEC Holdings Ltd. ("GTEC"), a Canadian licensed producer of handcrafted and high-quality cannabis (the "GTEC Agreement"). According to the GTEC Agreement, Focus will import GTEC's high-THC medical cannabis inflorescence into Israel to be sold under the IMC brand. With the arrival of these commercial shipments, the Company will launch a new category of imported premium indoor medical cannabis products under its well-established brand. The import of the Canadian-grown high-THC strains from GTEC's subsidiary, Grey Bruce Farms Incorporated ("GBF"), is expected to commence in Q2 2021, subject to fulfilling all regulatory requirements in relation to such import, including compliance with MOH regulations and receipt of a valid export license from Health Canada. According to the GTEC Agreement, Focus will purchase a minimum quantity of 500kg of high-THC medical cannabis inflorescence from GBF and will be the exclusive recipient of GTEC cannabis products in the Israeli market for a period of 12 months from the date that the first shipment of GTEC products arrives in Israel (the "Exclusive Term"). The Exclusive Term can be extended under the terms of the GTEC Agreement by an additional 6 months.
|
IM Cannabis Corp. |
||
|
Management’s Discussion and Analysis |
4. On March 12, 2021, the Company filed a preliminary short form base shelf prospectus (the "Preliminary Shelf Prospectus") with the securities commissions or similar securities regulatory authorities in each of the provinces and territories of Canada (the "Securities Commissions"), and on March 15, 2021, the Company filed a corresponding shelf registration statement on Form F-10, with the SEC under the Multijurisdictional Disclosure System ("MJDS") established between Canada and the United States.
5. On March 12, 2021, Adjupharm entered into a supply agreement with Northern Green Canada Inc. ("NGC") (the "NGC Supply Agreement"). Under the terms of the NGC Supply Agreement, NGC will provide Adjupharm with three new strains of medical cannabis products, to be distributed under the IMC brand to German pharmacies pursuant to Adjupharm's distribution agreements with its German distribution partners. Shipments from NGC are expected to commence in Q2 2021.
6. On March 18, 2021, the Company acquired all of Trichome's issued and outstanding shares (the "Trichome Shares") and closed the Trichome Transaction that was previously announced on December 30, 2020. Pursuant to the terms of the Trichome Transaction, former holders of Trichome Shares and former holders of Trichome convertible instruments (the "Trichome Securityholders") received 0.24525 of a Common Share for each Trichome Share held and each in-the-money convertible instrument of Trichome. As a result of the Trichome Transaction, a total of 10,104,901 Common Shares were issued to the Trichome Securityholders, resulting in former Trichome Securityholders holding approximately 20.06% of the total number of issued and outstanding Common Shares immediately after closing. In addition, 100,916 Common Shares were issued to financial advisors for advisory fees in connection with the Trichome Transaction.
7. On March 29, 2021, Adjupharm entered into a supply agreement with MediPharm Labs Corp. ("MediPharm Labs") for certain medical cannabis extract products to be delivered by MediPharm Labs over an initial two-year term with an automatic two-year extension period.
8. On March 31, 2021, in connection with the Preliminary Shelf Prospectus, the Company filed a final short form base shelf prospectus (the "Final Shelf Prospectus") with the Securities Commissions and a corresponding shelf registration statement on Form F-10 (the "Registration Statement") with the SEC. The Final Shelf Prospectus and the Registration Statement enable the Company to offer up to USD 250,000 (or its equivalent in other currencies) of Common Shares, warrants, subscription receipts, debt securities, units (collectively, the "Qualified Securities"), or any combination of such Qualified Securities from time to time, during the 25-month period that the Final Shelf Prospectus is effective. The specific terms of any offering under the Final Shelf Prospectus and the intended use of the net proceeds will be established in a prospectus supplement, which will be filed with the Securities Commissions and the SEC in connection with any such offering.
9. During March 2021, Adjupharm entered into two supply agreements with supply partners in China, under which Adjupharm shall buy COVID-19 rapid antigen test kits. Concurrently, Adjupharm entered into several resale agreements with reseller partners in Germany, under which Adjupharm shall sell the COVID-19 antigen test kits supplied from the China-based suppliers, to be distributed to pharmacies and retailers in Germany.
|
IM Cannabis Corp. |
||
|
Management’s Discussion and Analysis |
10. On April 1, 2021, the Company entered into a definitive agreement to acquire MYM and its licensed producer subsidiary Highland Grow Inc., pursuant to a plan of arrangement to be completed under the OBCA. Under the terms of the MYM Transaction, the shareholders of MYM will receive 0.022 Common Shares for each common share of MYM. Upon completion of the MYM Transaction, MYM former shareholders will own approximately 14.5% of the Company. The completion of the MYM Transaction is expected to occur before the end of 2021, and it will be subject to required court, securityholder and regulatory approvals.
Company Outlook
In Israel, the Company, through the Commercial Agreements, continues to expand the IMC brand recognition, and supply, in association with Focus, the growing medical cannabis market in Israel with products bearing the IMC brand. With the expected high growth of the Israeli medical cannabis market, the Company is well positioned to reap the benefits of its long-term presence and strong brand from this market expansion as it expects increases in both revenues and profitability. Additionally, the Group is focused on diversifying its product portfolio with premium and super premium medical cannabis products, leveraging its North American acquisition strategy that is expected to result in additional opportunities to export premium cannabis products to both Israel and Germany.
The Company's objective within Europe is to capitalize on the increasing demand for medical cannabis products and to bring the well-established IMC brand and its product portfolio to European patients. The Company's operating track record, accumulation of data and brand reputation in Israel is a competitive advantage in gaining traction within the German and European markets and building support among physicians who prescribe medical cannabis products.
In Canada, the Company is focused on continuing with an aggressive and accretive acquisition strategy focusing on attractively valued and highly synergistic targets.
Following the successful completion of the Trichome Transaction on March 18, 2021, IMCC's global platform now includes the adult-use recreational cannabis market, in addition to its established distribution channels for medical cannabis in Israel through Focus and in Germany through Adjupharm. Additionally, the Company's senior management team now includes extensive experience in acquisitions and restructuring to capitalize on consolidating a targeted list of attractively valued and highly synergistic assets.
Furthermore, the Company is planning to leverage TJAC's premium indoor cultivation capability to meet growing demand for premium cannabis under IMC's established international distribution platform.
The Company believes that successful completion of the MYM Transaction will enhance IMCC's focus on premium and super premium branded cannabis products in Canada. Furthermore, with coast-to-coast distribution, including a strong leadership position in eastern Canada, Highland Grow will expand the Company's distribution capabilities, fast track the entrance of JWC (expected soon to be relaunched as "Wagners") into new markets, and is expected to drive significant incremental revenue and EBITDA growth.
|
IM Cannabis Corp. |
||
|
Management’s Discussion and Analysis |
Overview of Financial Performance
|
|
For the year ended December 31, |
|
For the three months ended December 31, |
|
|||
|
|
2020 |
|
2019 |
|
2020 |
2019 |
|
|
Revenues |
$ 15,890 |
|
$ 9 ,074 |
|
$ 4,900 |
$ 2,479 |
|
|
Gross profit before fair value impacts in cost of sales |
$ 8,809 |
|
$ 4,313 |
|
$ 2,791 |
$ 881 |
|
|
Gross margin before fair value impacts in cost of sales (%) |
55 % |
|
48% |
|
57% |
36% |
|
|
Operating loss |
$ (8,245) |
|
$ (10,275) |
|
$ (6,383) |
$ (6,222) |
|
|
Net Income (Loss) |
$ (28,734) |
|
$ (7,419) |
|
$ (19,976) |
$ 1,693 |
|
|
Net Income (Loss) per share attributable to equity holders of the Company - Basic and Diluted (in CAD) |
$ (0.74) |
|
$ (0.23) |
|
$ (0.50) |
$ 0.08 |
|
The Overview of Financial Performance includes reference to "gross margin", which is a non-IFRS financial measure. Non-IFRS measures are not recognized measures under IFRS, do not have a standardized meaning prescribed by IFRS and are therefore unlikely to be comparable to similar measures presented by other companies. The Company defines gross margin as the difference between revenue and cost of goods sold divided by revenue (expressed as a percentage), prior to the effect of a fair value adjustment for inventory and biological assets.
|
IM Cannabis Corp. |
||
|
Management’s Discussion and Analysis |
Operational Results - Medical Cannabis
|
|
|
For the year ended December 31, |
|
For the three months December 31, |
||||
|
|
|
2020 |
|
2019 |
|
2020 |
|
2019 |
|
Average net selling price of dried cannabis (per Gram) |
|
$ 5.75 |
|
$ 3.39 |
|
$ 5.51 |
|
$ 4.50 |
|
Quantity harvested (in Kilograms) |
|
4,564 |
|
2,351 |
|
1,610 |
|
863 |
|
Quantity sold (in Kilograms) |
|
2,586 |
|
2,180 |
|
1,079 |
|
482 |
Review of Operations for the year ended December 31, 2020 and 2019
Revenues
The Group operates in one reporting segment. The main revenues of the Group are generated from sales of medical cannabis products to customers in Israel.
Revenues for the year ended December 31, 2020 and 2019 were $15,890 and $9,074, respectively, representing an increase of $6,816 or 75%. Total product sold for the year ended December 31, 2020 was 2,586kg at an average selling price of $5.75 per gram compared to 2,180kg at an average selling price of $3.39 per gram for the year ended December 31, 2019.
Revenues for the three months ended December 31, 2020 and 2019 were $4,900 and $2,479, respectively, representing an increase of $2,421 or 98%. The increase in revenues for the three months ended December 31, 2020 is attributable to deliveries made under the Focus' sales agreements to pharmacies, as well as to the increased average selling price of $5.51 per gram, compared to a $4.50 average selling price per gram for the three months ended December 31, 2019.
Cost of Revenues
The cost of revenues includes production, testing, shipping and sales related costs. At harvest, the biological assets are transferred to inventory at their fair value which becomes the deemed cost for the inventory. Inventory is later expensed to the cost of sales when sold. Direct production costs are expensed through the cost of sales.
The cost of revenues for the year ended December 31, 2020 and 2019 were $7,081 and $4,761, respectively, representing an increase of $2,320 or 49%. Cost of revenues for the three months ended December 31, 2020 and 2019 were $2,109 and $1,599, respectively, representing an increase of $510 or 32%. Most of the cost of revenues were comprised of production works, utilities, salary expenses and import costs, as well as certain adjustments made by the Company in order to adhere to the MOH's new regulation. Focus expects net cost of sales to vary from quarter to quarter based on the number of pre-harvest plants, after harvest plants, the strains being grown and technological progress in the trimming machines.
|
IM Cannabis Corp. |
||
|
Management’s Discussion and Analysis |
Gross Profit
Included in the Company's calculation of gross profit are the following:
• production costs (current period costs that are directly attributable to the cannabis growing and harvesting process);
• a fair value adjustment on sale of inventory (the change in fair value associated with biological assets that were transferred to inventory upon harvest);
• a fair value adjustment on growth of biological assets (the estimated fair value less cost to sell of biological assets as at the reporting date).
Included in gross profit is the net change in fair value of biological assets, inventory expensed and production costs. Biological assets consist of cannabis plants at various after-harvest stages which are recorded at fair value less costs to sell after harvest.
Gross profit for the year ended December 31, 2020 and 2019 was $10,468 and $3,929, respectively, representing an increase of $6,539 or 166%. For the three months ended December 31, 2020 and 2019 gross profit (loss) was $507 and $(274), respectively, representing an increase of $781 or 285%. This increase is attributed mainly to the cannabis price increase described above as well as the growth in high THC sales that enjoy from higher margins. Gross profit included gains from unrealized changes in fair value of biological assets and realized fair value adjustments on inventory sold of $1,659 and $(384) for the year ended December 31, 2020 and 2019, respectively. For the three months ended December 31, 2020 and 2019, total fair value adjustments were $(2,284) and $(1,154), respectively.
Expenses
General and Administrative
General and administrative expenses for the year ended December 31, 2020 and 2019 were $11,413 and $6,422, respectively, representing an increase of $4,991 or 78%. For the three months ended December 31, 2020 and 2019, general and administrative expenses were $4,190 and $1,297, respectively, representing an increase of $2,893 or 223%. The increase in the general and administrative is mainly attributable to the growing corporate activity in Israel and Germany, as professional services derived from legal fees and other consulting services in relation to the NASDAQ listing and M&A processes in the amount of $4,607, salaries and bonuses to employees in relation to the Company's performances in the amount of $4,394, and insurance costs in the amount of $701.
Selling and Marketing
Selling and marketing expenses for the year ended December 31, 2020 and 2019 were $3,782 and $1,240, respectively, representing an increase of $2,542 or 205%. For the three months ended December 31, 2020, selling and marketing expenses were $1,448, compared to $276 for the three months ended December 31, 2019, representing an increase of $1,172 or 425%. The increase in the selling and marketing expenses was due to the Company's increased marketing efforts in Israel and brand launch in Germany as well as increased distribution expenses relating to the increase in sales.
|
IM Cannabis Corp. |
||
|
Management’s Discussion and Analysis |
Research and Development
Research and development expenses for the year ended December 31, 2020 and 2019 were $136 and $233, respectively, representing a decrease of $97 or 42%. For the three months ended December 31, 2020 and 2019, research and development expenses were $1 and $32, respectively, representing a decrease of $31 or 97%. The decrease for the year ended December 31was primarily associated with the COVID-19 pandemic, which caused delays in new projects in Greece and Portugal.
Share-Based Compensation
Share-based compensation expense for the year ended December 31, 2020 and 2019 was $3,382 and $2,677, respectively, representing an increase $705 or 26%. For the three months ended December 31, 2020 and 2019, share-based compensation expense was $1,251 and $712, respectively, representing an increase of $539 or 76% which derived from the leave of several employees. The increase was mainly due to the grant of new incentive stock options ("Options") on September 9, 2020 and the increase in the Company's share price which led to increase in the fair value adjustment of consultants' options.
Financing
Financing income (expense), net, for the year ended December 31, 2020 and 2019 was $(20,227) and $2,946, respectively, representing a decrease of $23,173 or 787%. For the three months ended December 31, 2020, financing income (expense) was $(14,252) and $7,548, respectively, representing a decrease of $21,800 or 289%. The change was mainly due to the valuation update of the Warrants, which was affected by the Company's increased share price.
Depreciation and Amortization
Depreciation and amortization expenses for year ended December 31, 2020 and 2019 were $930 and $620, respectively, representing an increase of $310 or 50%. For the three months ended December 31, 2020 and 2019, depreciation and amortization expenses were $259 and $184, respectively, representing an increase of $75 or 41%. Depreciation and amortization expenses are impacted by the adoption of IFRS 16, renewal of Focus' greenhouses and Focus' purchase of additional production equipment, as well as the amortization of intangible assets following the acquisition of Adjupharm.
Net Income (Loss)
Net loss for the year ended December 31, 2020 and 2019 was $(28,734) and $(7,419), respectively, representing a net loss increase of $(21,315) or 287%. For the three months ended December 31, 2020 and 2019, net income (loss) was $(19,976) and $1,693, respectively, representing a decrease of $21,669 or 1,280%. The net decrease related to factors impacting net income from operations described above, and finance expenses driven by revaluation of Warrants in the amount of $20,155, which were recorded against liability on the grant day and were re-evaluated at December 31, 2020 through profit or loss.
|
IM Cannabis Corp. |
||
|
Management’s Discussion and Analysis |
Loss per Share
Basic loss per share is calculated by dividing the net profit attributable to common shareholders of the Company by the weighted average number of common shares outstanding during the period. Diluted profit per Common Share is calculated by adjusting the earnings and number of Common Shares for the effects of dilutive Warrants and other potentially dilutive securities. The weighted average number of Common Shares used as the denominator in calculating diluted profit per Common Share excludes unissued Common Shares related to Options as they are antidilutive. Basic and diluted loss per Common Share for the twelve and three months ended December 31, 2020 were ($0.74) and ($0.50) per Common Share, respectively.
Total Assets
Total assets as at December 31, 2020 were $38,116, compared to $30,894 as at December 31, 2019, representing an increase of $7,222 or 23%. This increase was primarily due to the completion of the private placement offering of Subscription Receipts, in which Finco, a subsidiary of the Company, raised approximately $20,433. During the year ended on December 31, 2020, the Company received $6,990 proceeds from the exercise of Warrants, Compensation Options and Options, out of which, $6,032 was received for the 2018 Warrants and Compensation Options. A total of 12,610,925 Warrants and Compensation Options were exercised, out of which, 12,350,795 were the 2018 Warrants and Compensation Options, representing 92.1% of the total quantity of Warrants and Compensation Options, at a price of $0.50 per 2018 Warrant and $0.40 per 2018 Compensation Option. The Company used part of the proceeds from the warrant exercises for its operating activities where trade receivables and inventories increased by $3,691 and $2,948, respectively, during 2020. Investing activities in property, plant and equipment and investments increased by $2,140 and $1,429, respectively, during 2020.
Total Liabilities
Total liabilities as at December 31, 2020 were $25,506, compared to $4,785 at December 31, 2019, representing an increase of $20 ,721 or 433%. The increase was primarily due to an increase of $16,343 in Warrants liability, and a slight increase in trade payables, other payables and deferred tax liability.
Intangible Assets
On March 15, 2019, IMC Holdings acquired Adjupharm, a licensed EU-GMP producer with wholesale, narcotics handling and import/export licenses for medical cannabis. As part of its global expansion and penetration plan into the European market, IMC acquired 100% of Adjupharm's issued and outstanding shares for €924 (approximately $1,400).
Through the acquisition of Adjupharm, the Company recognized $1,287 in intangible assets and goodwill. The goodwill arising on the acquisition was attributed to the expected benefits from the synergies of the combination of the activities of the Company and Adjupharm.
The goodwill recognized is not expected to be deductible for income tax purposes.
The Company recognized and updated the fair value of the assets acquired and liabilities assumed in the business combination according to a final valuation made by an external valuation specialist.
|
IM Cannabis Corp. |
||
|
Management’s Discussion and Analysis |
Liquidity and Capital Resources
For the year ended December 31, 2020, the Company generated revenues of $15,890 and received $6,990 in proceeds from the exercises of Warrants, Compensation Options and Options. Prior to receiving these proceeds, the Company financed its operations and met its capital requirements primarily through the October 2019 equity financing, upon the Reverse Takeover Transaction and listing on the CSE. The Company's objectives when managing its liquidity and capital resources are to generate enough cash to fund the Company's operating and working capital requirements as well as its strategy of being listed on NASDAQ. The Company believes that the generated cash flow form working capital in the different jurisdictions on which it operates, as well as the additional expected exercises of Warrants and future financing rounds will meet all of its future requirements. In evaluating its capital requirements, including the impact, if any, on the Company from the COVID-19 pandemic, and the ability to fund the execution of its strategy, the Company believes it has adequate availability to meet its working capital and other operating requirements, fund growth initiatives and capital expenditures, settle its liabilities, and repay scheduled principal and interest payments on debt for at least the next twelve months.
The Company has ensured that it has access to public capital markets through its CSE listing, and continues to review and pursue selected external financing sources to ensure adequate financial resources. These potential sources include, but are not limited to (i) obtaining financing from traditional or non-traditional investment capital organizations and (ii) obtaining funding from the sale of the Company's securities. There can be no assurance that we will gain adequate market acceptance for our products or be able to generate sufficient positive cash flow to achieve our business plans. We expect to continue funding these purchases with our available cash, cash equivalents and short-term investments. Therefore, we are subject to risks including, but not limited to, our inability to raise additional funds through financings to support our continued development, including capital expenditure requirements, operating requirements and to meet our liabilities and commitments as they come due. As at December 31, 2020, the Company had a working capital surplus of $20,874, compared to working capital of $21,682 as at December 31, 2019. The decrease in working capital of $808 was primarily due to increase in the current liabilities. As of December 31, 2020, the Company had an unaudited cash balance of $8,885 and no debt.
As at December 31, 2020, the Group's financial liabilities consisted of accounts payable and other accounts payable which have contractual maturity dates within one year. The Group manages its liquidity risk by reviewing its capital requirements on an ongoing basis. Based on the Group's working capital position at December 31, 2020, management considers liquidity risk to be low.
As at December 31, 2020, the Group has identified the following liquidity risks related to financial liabilities:
|
|
|
Less than |
|
1 to 5 |
|
6 to 10 |
|
|
|
|
|
|
|
|
|
|
|
Lease liabilities |
|
$ 232 |
|
$ 547 |
|
$ 515 |
|
The maturity profile of the Company's other financial liabilities (trade payables, other account payable and accrued expenses, and warrants) as of December 31, 2020 are less than one year.
|
IM Cannabis Corp. |
||
|
Management’s Discussion and Analysis |
The Annual Financial Statements have been prepared on the basis of accounting principles applicable to a going concern, which assumes that the Company will continue in operation for the foreseeable future and will be able to realize its assets and discharge its liabilities in the normal course of operations. The Annual Financial Statements do not include any adjustments to the amounts and classification of assets and liabilities that would be necessary should the Company be unable to continue as a going concern. Such adjustments could be material.
Share Capital
The Company's authorized share capital consists of an unlimited number of Common Shares without par value, 159,063,128 of which were issued and outstanding as of December 31, 2020.
The Common Shares confer upon their holders the right to participate in the general meeting with each Common Share having one voting right on all matters. The Common Shares also allow holders to receive dividends if and when declared and to participate in the distribution of surplus assets in the case of liquidation of the Company.
Operating, Financing and Investing Activities
The following table highlights the Company's cash flows for the twelve and three months ended December 31, 2020 and 2019:
| For the year ended December 31, |
For the three months ended December 31, |
|||||||||||
| Net cash provided by (used in): | 2020 | 2019 | 2020 | 2019 | ||||||||
| Operating activities | $ | (7,919 | ) | $ | (5,959 | ) | $ | (535 | ) | $ | (3,113 | ) |
| Investing activities | $ | (4,075 | ) | $ | (3,775 | ) | $ | (838 | ) | $ | (1,539 | ) |
| Financing activities | $ | 6,740 | $ | 17,051 | $ | 502 | $ | 17,781 | ||||
| Effect of foreign exchange | $ | 213 | $ | (982 | ) | $ | 19 | $ | (534 | ) | ||
| Decrease in cash | $ | (5,041 | ) | $ | 6,335 | $ | (852 | ) | $ | 12,595 | ||
Operating activities used cash of $7,919 and $535 for the twelve and three months ended December 31, 2020, respectively, as compared to $5,959 and $3,113 for the twelve and three months ended December 31, 2019, respectively. This variance is primarily due to increase in the business activities of the Company including corporate expenses for salaries, professional fees and marketing expenses in Israel and Germany. In the three months ended December 31, 2020, cash was primarily used to increase operating activities in connection with the Company's operations in Germany and the preparation of its Israeli operations to deliver medical cannabis under the Focus' sales agreements to pharmacies.
|
IM Cannabis Corp. |
||
|
Management’s Discussion and Analysis |
Investing activities used cash of $4,075 and $838 for the twelve and three months ended December 31, 2020, respectively, as compared to $3,775 and 1,539 for the twelve and three months ended December 31, 2019, respectively. In the three months ended December 31, 2020, cash was used primarily for the purchase of production equipment for Focus and Adjupharm as well as for investment in Xinteza.
Financing activities provided by cash of $6,740 and $502 for the twelve and three months ended December 31, 2020, respectively, as compared to $17,051 and $17,781 for the twelve and three months ended December 31, 2019, respectively. Most of the cash provided by finance activities in the three and twelve months ended December 31, 2020 were derived from the $6,378 in gross proceeds from the exercise of Warrants, Compensation Options and $612 from the exercise of Options, as well as from the repayment of a $250 lease liability and lease liability interest.
Selected quarterly financial information
|
For the three months ended |
December 31, |
September 30, |
June 30, |
March 31, |
|
Revenues |
$ 4,900 |
$ 5,893 |
$ 3,757 |
$ 1,340 |
|
Net income (Loss) |
$ (19,976) |
$ 738 |
$ (9,696) |
$ 200 |
|
Basic and diluted net income (Loss) per share (in CAD): |
$ (0.50) |
$ 0.00 |
$ (0.52) |
$ (0.00) |
|
For the three months ended |
December 31, |
September 30, |
June 30, |
March 31, |
|
Revenues |
$ 2,479 |
$ 2,326 |
$ 2,314 |
$ 1,955 |
|
Net income (Loss) |
$ 1,693 |
$ (1,915) |
$ (610) |
$ (6,591) |
|
Basic and diluted net income (Loss) per share (in CAD): |
$ 0.08 |
$ (0.04) |
$ (0.04) |
$ (0.24) |
On a quarterly basis, apart from the results of the first quarter of 2020 which were considered by the Company as preparation period for successful delivery of medical cannabis products under the Focus' sales agreement to pharmacies, and the results of the fourth quarter of 2020 which were affected by the COVID-19 outcomes on the German market, the Company has consistently increased revenues, which reflects the Company's expansion strategy.
|
IM Cannabis Corp. |
||
|
Management’s Discussion and Analysis |
Metrics and Non-IFRS Financial Measures
This MD&A makes reference to certain non-IFRS financial measures including "Gross Margin", "EBITDA", and "Adjusted EBITDA". These measures are not recognized measures under IFRS and do not have a standardized meaning prescribed by IFRS and are therefore unlikely to be comparable to similar measures presented by other companies. Rather, these measures are provided as additional information to complement those IFRS measures by providing further understanding of our results of operations from management's perspective. Accordingly, these measures should neither be considered in isolation nor as a substitute for analysis of our financial information reported under IFRS.
Management defines EBITDA as income earned or lost from operations, as reported, before interest, tax, depreciation and amortization. Adjusted EBITDA is defined as EBITDA, adjusted by removing other non-recurring or non-cash items, including the unrealized change in fair value of biological assets, realized fair value adjustments on inventory sold in the period, share-based compensation expenses, and revaluation adjustments of financial assets and liabilities measured on a fair value basis. Management believes that Adjusted EBITDA is a useful financial metric to assess its operating performance on a cash adjusted basis before the impact of non-recurring or non-cash items. The Company defines gross margin as the difference between revenue and cost of goods sold divided by revenue (expressed as a percentage), prior to the effect of a fair value adjustment for inventory and biological assets.
These non-IFRS financial measures can provide investors with supplemental measures of our operating performance and thus highlight trends in our core business that may not otherwise be apparent when relying solely on IFRS measures. We also believe that securities analysts, investors and other interested parties frequently use non-IFRS financial measures in the evaluation of issuers. Our management also uses these non-IFRS financial measures in order to facilitate operating performance comparisons from period to period, to prepare annual operating budgets and forecasts and to determine components of management compensation. As required by Canadian securities laws, we reconcile these non-IFRS financial measures to the most comparable IFRS measures.
|
IM Cannabis Corp. |
||
|
Management’s Discussion and Analysis |
|
|
For the year ended |
|
For the three months ended |
||||
|
|
2020 |
|
2019 |
|
2020 |
|
2019 |
|
Operational Loss |
$ (8,245) |
|
$(10,275) |
|
$ (6,383) |
|
$ (6,222) |
|
Depreciation & Amortization |
$ 930 |
|
$ 601 |
|
$ 258 |
|
$ 165 |
|
EBITDA |
$ (7,315) |
|
$ (9,674) |
|
$ (6,125) |
|
$ (6,057) |
|
|
|
|
|
|
|
|
|
|
IFRS Biological assets fair value adjustments, net |
$ (1,659) |
|
$ 384 |
|
$ 2,284 |
|
$ 1,154 |
|
Share-based payments |
$ 3,382 |
|
$ 2,677 |
|
$ 1,251 |
|
$ 713 |
|
Non-recurring costs related to the RTO |
$ - |
|
$ 3,632 |
|
$ - |
|
$ 3,632 |
|
Costs related to the NASDAQ listing |
$ 175 |
|
$ - |
|
$ 175 |
|
$ - |
|
Other Non-recurring costs |
$ 520 |
|
$ 1,167 |
|
$ (5) |
|
$ - |
|
Adjusted EBITDA (Non-IFRS) |
$ (4,897) |
|
$ (1,814) |
|
$ (2,420) |
|
$ (558) |
Adjusted EBITDA for the year ended December 31, 2020 and 2019 was $(4,897) and $(1,814), respectively, representing a decrease of $3,083. Adjusted EBITDA for the three months ended December 31, 2020 and 2019 was $(2,420) and $(558), respectively, representing a decrease of $1,862. The Company's Adjusted EBITDA for the year and three month period ended December 31, 2020 decreased as the Group increased its corporate expenses due to extensive efforts in fulfilling its business objectives while starting deliveries under its sales agreements to pharmacies and distribution partners, as applicable; other costs, including increased salaries expenses, increased selling and marketing expenses, mainly in Germany, professional services relating to M&A efforts, and NASDAQ listing expenses.
Contingent Liabilities and Commitments
(i) Rental Liabilities
In August 2010, Focus signed an agreement with a farmer, located in the south of Israel (the "Farmer"), according to which, Focus and the Farmer agreed to jointly operate an area of 7,000 square meters (the "Facility") for the cultivation and processing of medical cannabis (the "Venture"). For the purpose of this Venture, the parties agreed to operate under the operation of Focus. As part of the agreement, 26% of the share capital of Focus was allocated to the Farmer.
On December 1, 2016, Focus signed an additional agreement with the Farmer, according to which Focus agreed to operate an additional area of 6,000 square meters for the cultivation and processing of medical cannabis, under the operation of Focus.
|
IM Cannabis Corp. |
||
|
Management’s Discussion and Analysis |
On October 29, 2019, Focus signed with the Farmer an additional agreement, according to which Focus will operate an additional area of 7,500 square meters for the cultivation and processing of medical cannabis, under the framework of Focus.
(ii) Class Action - T.Z. 8394-11-16
On November 3, 2016, a motion was filed for approval of a class action against Focus and seven other Israeli cannabis growers (collectively, the "Growers"), for: (1) alleged use of chemical pesticides in the cannabis growing process, in contradiction to the Plant Protection Regulations and to the Protection of Public Health Regulations (Food) (Residues of Pesticides), and the misleading of their customers, thus violating the Consumer Protection Law; (2) selling cannabis product with lower concentration of active ingredients than publicized; and (3) marketing products in defective packaging - allegedly causing violation of autonomy and unjust enrichment. The personal suit sum for every class member stands at NIS 5,000 ($2). The total amount of the class action suit was estimated at NIS 133,000,000 ($50,633).
On January 4, 2021, the Court denied the motion, determining that the applicants had not proved an evidentiary basis for their motion.
(iii) Class Action T.Z. 35676-08-19
On August 19, 2019, a motion was filed for approval of a class action (the "Motion") against 17 companies (the "Companies") operating in the field of medical cannabis in Israel, including Focus. The applicant's argument is that the Companies did not accurately mark the concentration of active ingredients in their products. The personal suit sum for every class member stands at NIS 15,585 ($5,900 ) and the total amount of the class action suit is estimated at NIS 686,000($2 61 ,000). On June 2, 2020, the Companies submitted their response to the Motion. The Companies argue in their response that the threshold conditions for approval of a class action were not met, since there is no reasonable possibility that the causes of action in the Motion will be decided in favor of the class group. On July 3, 2020, the applicant submitted his response to the Companies' response. On July 5, 2020 the applicant was absent from the hearing. As a result, on July 23, 2020, the Companies filed an application for a ruling of expenses, which received a response from the applicant on August 12, 2020, asking to decline this request. On September 21, 2020, the court ruled that the applicant would pay the Companies' expenses amount of 750 NIS. Prehearing is set for July 14, 2021.
At this preliminary stage, based on the opinion of its legal counsel, Focus' management cannot, assess the chances of approval of the Motion. Therefore, no provision has been recorded in respect thereof.
(iv) Supreme Court of Justice 2335/19
On October 6, 2019, Focus received a decision regarding a petition that was filed against the MOH, concerning the new regulatory framework of the cannabis market and demanding that the court resolve as follows:
• that the MOH immediately suspend the implementation of the new regulation that harms, disproportionally, the medical cannabis patients;
• that the implementation of the new regulation, as is, would cause violation of constitutional rights of the medical cannabis patients; and
|
IM Cannabis Corp. |
||
|
Management’s Discussion and Analysis |
• that the MOH amend the flaws of the new regulation, prior to becoming effective, and to establish new regulations regarding labeling and use of pesticides.
According to the decision, Focus was attached to the proceedings and filed its response on November 12, 2019.
On March 8, 2020, the court decided to extend the validity of the interim injunction, so that the medical cannabis use licenses, which were extended under the decision, would continue to be valid until May 15, 2020, or 10 days after the date the MOH comes to a conclusion regarding the price control of medical cannabis products, whichever comes first, subject to another court decision.
The court also decided that if a further extension of the period of the interim injunction is granted beyond May 15, 2020, to the extent required, it would be subject to medical surveillance by the attending physician, that his details of which were included in the patient's existing use license.
On October 29, 2020, the respondents represented by the State Attorney's Office filed an update notice stating that the Appeals Committee unanimously decided against imposing price controls on medical cannabis products and that the Prices Committee would hold a follow-up hearing in four months. The respondents also requested to update the Court again in two months.
On November 25, 2020, the petitioner submitted their response to the respondents' update notice.
On March 25, 2021, the respondents represented by the State Attorney's Office filed an updating notice stating that the Prices Committee had come to a decision against imposing price controls on medical cannabis products. However, the Prices Committee announced that it will issue an RFI to the corporations engaged in the medical cannabis market and assess the market every six months. Following the aforementioned, the respondents represented by the State Attorney's Office believe that the appeal should be rejected and the interim injunction should be canceled. On April 13, 2021, three of the respondents filed a response to the court, requesting to reject the appeal and to cancel the interim injunction.
Based on the opinion of its legal counsel, Focus' management estimate that the chances of the petition are less than 50%.
(v) Class Action T.Z. 31805-10-19
On October 30, 2019, Focus was served with a motion for approval of a class action against it, the Medical Cannabis Unit of the MOH, and five other companies related to the cannabis market in Israel. The motion was filed in connection to a stopping of supplies of medical cannabis by way of direct supply. The legal causes alleged in the motion are the following: discrimination in violation of the Equal Rights for Persons with Disabilities Act, 1988 and a restrictive arrangement contrary to the Economic Competition Law, 1988. The motion argues that the class action group incurred damages in the amount of NIS 656,000 ($250,000). On November 11, 2020, Focus submitted its response to the motion and the pre-hearing was scheduled for March 21, 2021.
On March 14, 2021, the court denied the motion.
|
IM Cannabis Corp. |
||
|
Management’s Discussion and Analysis |
Off-Balance Sheet Arrangements
IMCC had no off-balance sheet arrangements as at December 31, 2020.
Transactions with Related Parties
The Company had no transactions with related parties outside of the group except those pertaining to transactions with key management personnel and shareholders in the ordinary course of their employment or directorship. Transactions with related parties for the sale of Focus due to the restructuring process were adjusted in the Company's consolidated financial statements following the application of IFRS 10.
Fourth Quarter
During the fourth quarter of 2020, the Group focused on establishing its presence in the Canadian market, announcing the acquisition of Trichome on December 30, 2020 and its wholly owned licensed producer, TJAC, thereby facilitating the entry into the Canadian adult-use market while also securing consistent supply of premium cannabis for the Group' operations in Israel and Germany. In Germany, the Company executed binding sales agreements with distribution partners, reaching more than 6,000 pharmacies. Also in the fourth quarter, the Company applied to list its shares on the NASDAQ.
Proposed Transactions
There are no proposed transactions as at the date of this MD&A that have not been disclosed.
Critical Accounting Estimates
In the process of applying the significant accounting policies, the Group has made the following judgments which have the most significant effect on the amounts recognized in the financial statements:
a. Judgments
Determining the fair value of share-based payment transactions
The fair value of share-based payment transactions is determined upon initial recognition by an acceptable option pricing model. The inputs to the model include share price, exercise price and assumptions regarding expected volatility, expected life of share option and expected dividend yield.
Discount rate for a lease liability
When the Company is unable to readily determine the discount rate implicit in a lease in order to measure the lease liability, the Company uses an incremental borrowing rate. That rate represents the rate of interest that the Company would have to pay to borrow over a similar term and with similar security, the funds necessary to obtain an asset of similar value to the right-of-use asset in a similar economic environment. When there are no financing transactions that can serve as a basis, the Company determines the incremental borrowing rate based on its credit risk, the lease term and other economic variables deriving from the lease contract's conditions and restrictions. In certain situations, the Company is assisted by an external valuation expert in determining the incremental borrowing rate.
|
IM Cannabis Corp. |
||
|
Management’s Discussion and Analysis |
b. Estimates and assumptions
The preparation of the financial statements requires management to make estimates and assumptions that have an effect on the application of the accounting policies and on the reported amounts of assets, liabilities, revenues and expenses. Changes in accounting estimates are reported in the period of the change in estimate.
The key assumptions made in the financial statements concerning uncertainties at the reporting date and the critical estimates computed by the Group that may result in a material adjustment to the carrying amounts of assets and liabilities within the next financial year are discussed below.
Assessment of going concern
The use of the going concern basis of preparation of the financial statements. At each reporting period, management assesses the basis of preparation of the financial statements. These financial statements have been prepared on a going concern basis in accordance with IFRS. The going concern basis of presentation assumes that the Company will continue its operations for the foreseeable future and be able to realize its assets and discharge its liabilities and commitments in the normal course of business.
In arriving at this determination, the Company has undertaken a thorough review of the Group's cash flow forecast and potential liquidity risks. Cash flow projections have been prepared which show that the Group's operations will be cash generative during the period of at least 12 months from the date of approval of the consolidated financial statements.
Biological assets and inventory
In calculating the value of the biological assets and inventory, management is required to make several estimates, including estimating the stage of growth of the cannabis up to the point of harvest, harvesting costs, selling costs, average or expected selling prices and list prices, expected yields for the cannabis plants, and oil conversion factors. The valuation of work-in-process and finished goods also requires the estimate of conversion costs incurred, which become part of the carrying amount for the inventory. The Company must also determine if the cost of any inventory exceeds its net realizable value, such as cases where prices have decreased, or inventory has spoiled or has otherwise been damaged. See Note 10 for further information.
Legal claims
In estimating the likelihood of legal claims filed against certain entities of the Group, the Company's management rely on the opinions of the respective legal counsel of each relevant entity of the Group. These estimations are based on each legal counsel's best professional judgment, taking into account the stage of proceedings and legal precedents in respect of the different issues. Since the outcome of the claims may be determined in courts, the results could differ from these estimations.
Deferred tax assets
Deferred tax assets are recognized for unused carryforward tax losses and deductible temporary differences to the extent that it is probable that taxable profit will be available against which the losses can be utilized. Significant management judgment is required to determine the amount of deferred tax assets that can be recognized, based upon the timing and level of future taxable profits, its source and the tax planning strategy.
|
IM Cannabis Corp. |
||
|
Management’s Discussion and Analysis |
Risk Factors
The Company has implemented risk management governance processes that are led by the board of directors, with the active participation of management, and updates its assessment of its business risks on an annual basis. Notwithstanding, it is possible that the Company may not be able to foresee all the risks that it may have to face. The market in which IMCC currently competes is complex, competitive and changing rapidly. Sometimes new risks emerge, and management may not be able to predict all of them or be able to predict how they may cause actual results to be different from those contained in forward looking statements. Readers of this MD&A should not rely upon forward looking statements as a prediction of future results.
The following risk factors have been identified by management:
(i) General Business Risk and Liability
Given the nature of the Company's business, it may from time to time be subject to claims or complaints from investors or others in the normal course of business. The legal risks facing the Company, its directors, officers, employees or agents in this respect include potential liability for violations of securities laws, breach of fiduciary duty or misuse of investors' funds. Some violations of securities laws and breach of fiduciary duty could result in civil liability, fines, sanctions, or the suspension or revocation of the Company's right to carry on its existing business. The Company may incur significant costs in connection with such potential liabilities.
(ii) Consolidation of Focus Financial Results under IFRS 10 and Maintenance of Common Control
The Company complies with IFRS 10, which applies a single consolidation model using a definition of "control" that requires an investor (as defined in IFRS 10) to consolidate an investee (as defined in IFRS 10) where: (i) the investor has power over the investee; (ii) the investor has exposure or rights to variable returns from involvement with the investee; and (iii) the investor can use its power over the investee to affect the amount of the investor's returns.
Subsequent to the IMC Restructuring, the Company analyzed the terms of the contractual agreements with Focus (including the Commercial Agreements and the Focus Agreement) in accordance with IFRS 10 to conclude whether it should continue to consolidate the accounts of Focus in its financial statements.
Under IFRS 10, consolidation occurs when an investor can exercise control over an investee. Control is achieved through voting rights or other evidence of power. Where there are no direct holdings, under IFRS 10, an investor (as defined in IFRS 10) should consider other evidence of power and ability to unilaterally direct an investee's (as defined in IFRS 10) relevant activities. In view of the contractual agreements and the guidance in IFRS 10, notwithstanding that the Company has no direct or indirect ownership of Focus, it has sufficient rights to unilaterally direct the relevant activities (a concept known as "de facto control"), mainly due to the following:
|
IM Cannabis Corp. |
||
|
Management’s Discussion and Analysis |
(a) the Company receives economic benefits from Focus (and the terms of the Commercial Agreements cannot be changed without the approval of the Company);
(b) the Company having the option to purchase the divested 74% interest in Focus held by Oren Shuster, the CEO, director and a promoter of the Company, and Rafael Gabay, a director and a promoter of the Company; Messrs. Shuster and Gabay each being a director of Focus (while Mr. Shuster concurrently being a director, officer and substantial shareholder of the Company and Mr. Gabay concurrently being a substantial shareholder of the Company); and
(c) the Company provides management and support activities to Focus through the Services Agreement.
Accordingly, under IFRS 10, the Company has "de facto control" over Focus, and therefore consolidates the financial results of Focus in the Company's financial statements.
Any failure of the Company or Messrs. Oren Shuster and Rafael Gabay to maintain "de facto control" over Focus as defined under IFRS 10 could alter the Company's consolidation model, potentially resulting in a material adverse effect on the business, results of operations and financial condition of the Company.
(iii) Ownership of Focus
There is a risk that regulatory authorities in Israel may view the Company as the deemed owner of more than 5% of Focus and/or determine that the Company is in contravention of Israeli cannabis regulations. Namely, prior approval of the IMCA is required for any shareholder owning 5% or more of an Israeli company licensed to engage in cannabis-related activities. Any contravention of Israeli cannabis regulations could jeopardize the good standing of the Focus License. Such a determination may adversely affect the Company's ability to conduct sales and marketing activities and could have a material adverse effect on the Company's business, operating results or financial condition.
(iv) Limited Operating History
The Company did not generate revenue from the sale of cannabis products until late 2019. The Company is therefore subject to many of the risks common to early-stage enterprises, including under-capitalization, cash shortages, limitations with respect to personnel, financial, and other resources and lack of revenues. There is no assurance that the Company will be successful in achieving a return on shareholders' investment and the likelihood of success must be considered in light of the early stage of operations.
(v) Negative Cash Flows
During the year ended December 31, 2020, the Company had negative cash flows from operating activities. Although the Company expects to generate positive cash flows from its future operating activities, there is no assurance that it will achieve this objective. If operational cash flows continue to be negative, the Company may be required to fund future operations with alternative financing options such as offerings of shares.
|
IM Cannabis Corp. |
||
|
Management’s Discussion and Analysis |
(vi) Additional Financing
There is no assurance that the Company will be able to secure the funds necessary to implement its strategies. Additional debt incurred by the Company from engagements such as major acquisitions may cause the Company's debt level to increase and result in difficulties in completing or negotiating future debt financings. Any triggering of credit defaults or failure to raise capital by the Company may cause significant delays in carrying out business objectives or result in a material adverse effect on the Company's business, financial condition, operational results and prospects.
(vii) No Control over Cannabis Operations of Investees
The Company's investees generally have the power to determine the manner in which their respective businesses are developed, expanded and operated. The interests of the Company and its investees may not always be aligned. As a result, the cash flows of the Company are dependent upon the activities of its investees, which creates the risk that at any time those investees may: (i) have business interests or targets that are inconsistent with those of the Company; (ii) take action contrary to the Company's policies or objectives; (iii) be unable or unwilling to fulfill their obligations under their agreements with the Company; or (iv) experience financial, operational or other difficulties, including insolvency, which could limit or suspend an investee's ability to perform its obligations under its agreements with the Company. The Company must rely on the accuracy and timeliness of the disclosure and information it receives from its investees. If the information contains material inaccuracies or omissions, the Company's ability to accurately forecast or achieve its stated objectives may be materially impaired. Failure to receive Company's entitlements pursuant to the agreements it has entered into may have a material adverse effect on the Company.
(viii) Compliance with Laws
The Company's and its investees' operations are subject to various laws, regulations and guidelines. The Company endeavors to and cause its investees to comply with all relevant laws, regulations and guidelines. However, there is a risk that the Company's and its investees' interpretation of laws, regulations and guidelines, including, but not limited to the Cannabis Act, the regulations thereunder and applicable stock exchange rules and regulations, may differ from each other, and the Company's and its investees' operations may not be in compliance with such laws, regulations and guidelines. In addition, achievement of the Company's business objectives is contingent, in part, upon compliance with regulatory requirements enacted by governmental authorities and, where necessary, obtaining regulatory approvals. The impact of regulatory compliance regimes, any delays in obtaining, or failure to obtain regulatory approvals required by the Company or its investees may significantly delay or impact the development of the Company's business and operations and could have a material adverse effect on the business, results of operations and financial condition of Company. Any potential noncompliance could cause the business, financial condition and results of operations of Company to be adversely affected. Further, any amendment to or replacement of the Cannabis Act or other applicable rules and regulations governing the activities of the investees may cause adverse effects to Company's operations. The risks to the business of Company and its investees associated with the decision to amend or replace the Cannabis Act and subsequent regulatory changes, could reduce the addressable market for the Company's or the investees' products and could materially and adversely affect the business, financial condition and results of operations of the Company.
The Company and its investees incur ongoing costs and obligations related to regulatory compliance. Failure to comply with applicable laws and regulations may result in enforcement actions thereunder, including orders issued by regulatory or judicial authorities causing operations to cease or be curtailed, and may include corrective measures requiring capital expenditures or remedial actions. Parties may be liable for civil or criminal fines or penalties imposed for violations of applicable laws or regulations. Amendments to current laws, regulations and permitting requirements, or more stringent application of existing laws or regulations, may have a material adverse impact on the Company and/or its investees, resulting in increased capital expenditures or production costs, reduced levels of cannabis production or abandonment or delays in the development of facilities which could have a material adverse effect on the business, results of operations and financial condition of the Company.
|
IM Cannabis Corp. |
||
|
Management’s Discussion and Analysis |
The introduction of new tax laws, regulations or rules, or changes to, or differing interpretations of, or application of, existing tax laws, regulations or rules in any of the countries in which the Company invests could result in an increase in the Company's taxes, or other governmental charges, duties or impositions. No assurance can be given that new tax laws, regulations or rules will not be enacted or that existing tax laws, regulations or rules will not be changed, interpreted or applied in a manner which could result in the Company's profits being subject to additional taxation or which could otherwise have a material adverse effect on the Company.
(ix) Regulation of the Cannabis Industry
The cannabis-related business and activities of the Group are heavily regulated in all jurisdictions where it carries on business. The Group's operations are subject to various laws, regulations and guidelines by governmental authorities, particularly the MOH and The Federal Opium Agency of Germany's Federal Institute for Drugs and Medical Devices (the "BfArM"), relating to the manufacture, marketing, management, transportation, storage, sale, pricing and disposal of medical cannabis and cannabis oil, and also including laws and regulations relating to health and safety, insurance coverage, the conduct of operations and the protection of the environment.
Laws and regulations, applied generally, grant government agencies and self-regulatory bodies broad administrative discretion over the activities of the Group, including the power to limit or restrict business activities as well as impose additional disclosure requirements on the Company's products and services. Achievement of the Group's business objectives are contingent, in part, upon compliance with regulatory requirements enacted by these governmental authorities and obtaining all regulatory approvals, where necessary, for the production and sale of its products.
The Group cannot predict the time required to secure all appropriate regulatory approvals for its products, or the extent of testing and documentation that may be required by governmental authorities. Any delays in obtaining, or failure to obtain regulatory approvals would significantly delay the development of markets and products and could have a material adverse effect on the business, results of operations and financial condition of the Company.
Failure to comply with the laws and regulations applicable to its operations may lead to possible sanctions including the revocation or imposition of additional conditions on licenses to operate the Group's business, the suspension or expulsion from a particular market or jurisdiction or of its key personnel, and the imposition of fines and censures. To the extent that there are changes to the existing laws and regulations or the enactment of future laws and regulations that affect the sale or offering of the Company's products or services in any way, this could have a material adverse effect on the business, results of operations and financial condition of the Group.
|
IM Cannabis Corp. |
||
|
Management’s Discussion and Analysis |
(x) Changes in Laws, Regulations and Other Guidelines
The Group's operations are subject to a variety of laws, regulations, and guidelines relating to the marketing, acquisition, manufacture, management, distribution (including import and export), transportation, storage, sale and disposal of medical cannabis products. The Group's operations are also subject to laws and regulations relating to health and safety, insurance coverage, the conduct of operations and the protection of the environment. While the Group is currently in compliance with all such laws, regulations and guidelines, any rulings to the contrary or any changes to such laws and regulations that are beyond the control of the Group could have a material adverse effect on the business, results of operations, financial condition and prospects of the Group.
(xi) Environmental and Employee Health and Safety Regulations
The Group's operations are subject to environmental and occupational safety laws and regulations in certain jurisdictions, concerning, among other things, emissions and discharges to water, air and land, the handling and disposal of hazardous and nonhazardous materials and wastes, and employee health and safety. The Group incurs ongoing costs and obligations related to compliance with environmental and employee health and safety matters. Any failure to comply or maintain compliance with environmental and occupational safety laws and regulations may result in additional costs for corrective measures, penalties or restrictions on manufacturing operations. In addition, changes in environmental, employee health and safety or other laws, more vigorous enforcement thereof or other unanticipated events could require extensive changes to the Group's operations or give rise to material liabilities, which could have a material adverse effect on the business, results of operations and financial condition of the Group. This is particularly relevant for Focus and Adjupharm as these entities engage in cannabis-related operations that may be more prone to environmental and employee safety issues. Any changes to current laws and regulations may require substantial investments by the Group in order to comply such changes. If substantial investments are required, there may be a material adverse effect on the Group's operations, financial condition and operating results.
(xii) Reliance on License and Permit Renewals
Focus and Adjupharm are dependent on certain licenses (together, the "Key Licenses"), respectively, and the need to maintain such Key Licenses in good standing. Failure to comply with the requirements or maintenance of any of the Key Licenses may have a material adverse effect on the business, financial condition and operating results of the Group. As of the date of this MD&A, Focus' license is valid until January 3, 2022 and the quantities for import under the Adjupharm licenses are valid until May 8, 2021. Although management of Focus and Adjupharm believe that they will continue to meet the requirements of the MOH and the BfArM, respectively, for the respective durations of the Key Licenses, there can be no guarantee that the MOH or BfArM will extend or renew any of the Key Licenses or, if any of the Key Licenses are extended or renewed, that they will be extended or renewed on the same or similar terms.
Should the MOH or BfArM not extend or renew any of the Key Licenses, or should it renew any of the Key Licenses on different terms or not allow for anticipated capacity increases, the business, financial condition, results of the operations and prospects of the Group may subject to a material adverse effect.
|
IM Cannabis Corp. |
||
|
Management’s Discussion and Analysis |
(xiii) Reliance on Other Business Licenses, Permits and Approvals
In addition to Focus' and Adjupharm's dependence on the Key Licenses mentioned above, the Group is also dependent on ancillary business licenses, permits and approvals granted by government authorities or other third parties in order to operate effectively including, without limitation, building permits, municipal permits, third-party licenses, and foreign trade licenses. Should the Group fail to maintain any of these licenses, permits and approvals, or should it fail to renew any of such licenses, permits and approvals on materially similar or more favorable terms, the business, financial condition and results of the operations of the Group may be subject to a material adverse effect.
(xiv) Reliance on Focus Facility
The Focus License is specific to the Focus Facility and both must remain in good standing for Focus to conduct the medical cannabis activities authorized thereunder. Adverse changes or developments affecting the Focus Facility, including but not limited to the failure to maintain all requisite regulatory and ancillary permits and licenses, the failure to comply with state or municipal regulations, or a breach of security, could have a material adverse effect on the Group's business, financial condition, results of operations and prospects.
In addition, any breach of the Focus Lease Agreement or any failure to renew the Focus Lease Agreement, on materially similar or more favorable terms, may have a material adverse effect on the Group's business, financial condition, results of operations and prospects, and could also have an impact on Focus' ability to continue operating under the Focus License or to renew the Focus License.
The Focus Facility is subject to state and municipal regulation and oversight, including the acquisition of all required regulatory and ancillary permits to conduct operations or undertake any construction. Any breach of regulatory requirements, security measures or other facility requirements, including any failure to comply with recommendations or requirements arising from inspections by government regulators at all levels, could also have an impact on Focus' ability to maintain the Focus Lease Agreement and/or keep the Focus Facility in good standing, and to continue operating under the Focus License or the prospect of renewing the Focus License.
The Focus Facility continues to operate with routine maintenance. Focus will bear many, if not all, of the costs of maintenance and upkeep of the Focus Facility, including replacement of components over time. Focus' operations and the Group's financial performance may be adversely affected if Focus is unable to keep up with maintenance requirements.
In December 2020, the municipal committee presiding over planning and construction in southern Israel (the "Construction Committee") advised Focus that it was the subject of certain allegations regarding inadequate permitting for construction relating to the Focus Facility (the "Construction Allegations"). Focus' shareholders and directors, including Oren Shuster and Rafael Gabay, received a summons and have testified before the Construction Committee. In January 2021, the MOH advised Focus that it had received a complaint of the same nature as the Construction Allegations (the "MOH Allegations"). Focus is fully cooperating with the ongoing investigations of both the Construction Committee and the MOH. As of the date of this MD&A, no formal legal proceedings have been commenced against any of Focus, Mr. Shuster or Mr. Gabay. In the event that formal legal proceedings in respect of the Construction Allegations and/or the MOH Allegations are launched, potential consequences of any negative outcome may include, but are not limited to: (i) criminal charges against any or all of Focus or Focus' shareholders and directors, including Mr. Shuster and Mr. Gabay; (ii) monetary penalties or fines; (iii) temporary or permanent suspension of the Focus License; and (iv) other consequences that may limit, in part or as a whole, Focus' operations under the Focus License. A negative outcome to the Construction Allegations or the MOH Allegations may have a material adverse effect on the business, results of operations and financial conditions of the Group.
|
IM Cannabis Corp. |
||
|
Management’s Discussion and Analysis |
(xv) Dependence on Senior Management
The success of the Company is dependent upon the contributions of senior management. The loss of any of these individuals, or an inability to attract, retain and motivate sufficient members of qualified senior management personnel could adversely affect its business. This risk is partially mitigated by the fact that the senior management team are significant shareholders in the Company.
(xvi) Competition in the Industry
There is potential that the Company will face intense competition from other companies, some of which can be expected to have more financial resources, industry, manufacturing and marketing experience than the Company. Because of the early stage of the industry in which IMCC operates, the Company expects to face additional competition from new entrants. If the number of users of medical cannabis in Israel increases, the demand for products will increase and the Company expects that competition will become more intense, as current and future competitors begin to offer an increasing number of diversified products and pricing strategies.
There is also the potential that the industry will undergo consolidation, creating larger companies that may have increased geographic scope. Increased competition by larger, better-financed competitors with geographic advantages could materially and adversely affect the business, financial condition and results of operations of the Company.
(xvii) Risks Inherent in the Agricultural Business
The Company's business, specifically as it pertains to its relationship with Focus, involves the growing of medical cannabis, which is an agricultural product. As such, the business is subject to the risks inherent in the agricultural business, such as pests, plant diseases and similar agricultural risks. Although Focus grows its products indoors under climate-controlled conditions, and carefully monitors the growing conditions with trained personnel, there can be no assurance that natural elements will not have a material adverse effect on the production of its products and results of operations of Focus.
(xviii) Restrictions on Sales and Marketing
The industry is in its early development stage and restrictions on sales and marketing activities imposed by the MOH, various medical associations, other governmental or quasi-governmental bodies or voluntary industry associations may adversely affect the Company's ability to conduct sales and marketing activities and could have a material adverse effect on the Company's business, operating results or financial condition.
(xix) Publicity or Consumer Perception
The Company believes the medical cannabis industry is highly dependent upon consumer perception regarding the safety, efficiency and quality of the medical cannabis produced. Consumer perception of the Company's products can be significantly influenced by scientific research or findings, regulatory investigations, litigation, media attention and other publicity regarding the consumption of medical cannabis products.
|
IM Cannabis Corp. |
||
|
Management’s Discussion and Analysis |
There can be no assurance that future scientific research, findings, regulatory proceedings, litigation, media attention or other research findings or publicity will be favorable to the medical cannabis market or any particular product, or consistent with earlier publicity.
Future research reports, findings, regulatory proceedings, litigation, media attention or other publicity that are perceived as less favorable than, or that question, earlier research reports, findings or publicity could have a material adverse effect on the demand for products bearing the Company's brand and the business, results of operations, financial condition and the Company's cash flows. The Company's dependence upon consumer perceptions means that adverse scientific research reports, findings, regulatory proceedings, litigation, media attention or other publicity, whether or not accurate or with merit, could have a material adverse effect on the Company, the demand for products bearing the Company's brand, and the business, results of operations, financial condition and cash flows of the Company.
Further, adverse publicity reports or other media attention regarding the safety, efficacy and quality of medical cannabis in general, or the Company's products specifically, or associating the consumption of medical cannabis with illness or other negative effects or events, could have such a material adverse effect. Such adverse publicity reports or other media attention could arise even if the adverse effects associated with such products resulted from consumers' failure to consume such products appropriately or as directed.
(xx) Reliance on Key Business Inputs
The Group's business is dependent on a number of key inputs and their related costs including raw materials and supplies related to its growing operations as well as electricity, water, and other utilities. Any significant interruption or negative change in the availability or economics of the supply chain for key inputs (e.g. rising energy costs) could materially impact the business, financial condition, and operating results of the Company. Any ability to secure required supplies and services or to do so on appropriate terms could also have a materially adverse opinion impact on the business, financial condition, and operating results of the Company.
(xxi) Sufficiency of Insurance
The Company maintains various types of insurance which may include product liability insurance (see "Potential Product Liability" below), errors and omission insurance, directors and officers insurance, trustees' insurance, property coverage, and, general commercial insurance. There is no assurance that claims will not exceed the limits of available coverage, that any insurer will remain solvent or willing to continue providing insurance coverage will sufficient limits or at a reasonable cost; or, that any insurer will not dispute coverage of certain claims due to ambiguities in the policies. A judgment against any member of the Company in excess of available coverage could have a material adverse effect on the Company in terms of damages awarded and the impact and reputation of the Company.
(xxii) Potential Product Liability
As IMCC derives a significant portion of its revenues from Focus, which is a manufacturer of products designed to be ingested or inhaled by humans. Focus products bearing the Company's brand face an inherent risk of exposure to product liability claims, regulatory action and litigation if such products are alleged to have caused significant loss or injury. In addition, the manufacture and sale of Focus products bearing the Company's brand involve the risk of injury or loss to consumers due to tampering by unauthorized third parties, product contamination, unauthorized use by consumers or other third parties. Previously unknown adverse reactions resulting from human consumption of Focus products bearing the Company's brand alone or in combination with other medications or substances could occur.
|
IM Cannabis Corp. |
||
|
Management’s Discussion and Analysis |
The Company may be subject to various product liability claims, including, among others, that products bearing IMC's brand caused injury, illness or loss, include inadequate instructions for use or include inadequate warnings concerning possible side effects or interactions with other substances. A product liability claim or regulatory action against the Company could result in increased costs, could adversely affect the Company's reputation with its clients and consumers generally, and could have a material adverse effect on our results of operations and financial condition of the Company.
There can be no assurances that the Company will be able to obtain or maintain product liability insurance on acceptable terms or with adequate coverage against potential liabilities. Such insurance is expensive and may not be available in the future on acceptable terms, or at all. The inability to obtain sufficient insurance coverage on reasonable terms or to otherwise protect against potential product liability claims could prevent or inhibit the commercialization of products bearing the Company's brand.
(xxiii) Potential General Litigation
The Company may become party to litigation from time to time in the ordinary course of business which could adversely affect its business. Should any litigation in which the Company become involved be determined against the Company, such a decision could adversely affect the Company's ability to continue operating and the market price for the Company's common shares and could use significant resources. Even if the Company is involved in litigation and wins, litigation can redirect significant Company resources.
(xxiv) Potential Product Recalls
Manufacturers and distributors of products are sometimes subject to the recall or return of their products for a variety of reasons, including product defects, such as contamination, unintended harmful side effects or interactions with other substances, packaging safety and inadequate or inaccurate labeling disclosure. If products bearing IMC's brand are recalled due to an alleged product defect or for any other reason, IMCC could be required to incur the unexpected expense of the recall and any legal proceedings that might arise in connection with the recall.
IMCC may lose a significant amount of sales and may not be able to replace those sales at an acceptable margin or at all. In addition, a product recall may require significant management attention.
Although the Company has detailed procedures in place for testing finished products, there can be no assurance that any quality, potency or contamination problems will be detected in time to avoid unforeseen product recalls, regulatory action or lawsuits. Additionally if one of the Company's significant brands were subject to recall, the image of that brand and the Company could be harmed. A recall for any one of the foregoing reasons could lead to decreased demand for the Company's products and could have a material adverse effect on the results of operations and financial condition of the Company. Additionally, product recalls may lead to increased scrutiny of IMCC's operations by the MOH or other regulatory agencies, requiring further management attention and potential legal fees and other expenses.
|
IM Cannabis Corp. |
||
|
Management’s Discussion and Analysis |
(xxv) Management of Growth
The Company may be subject to growth related risks including capacity constraints and pressure on its internal systems and controls. The ability of the Company to manage growth effectively will require it to continue to implement and improve its operational and financial systems and to expand, train and manage its employee base. If the Company is unable to deal with this growth, that may have a material adverse effect on the Company's business, financial condition, results of operation and prospects.
(xxvi) U.S. Operations
The Company and, to its knowledge, its investees, do not currently engage in any U.S. cannabis-related activities as defined in CSA Staff Notice 51-352. To date, the Company has caused its investees to only conduct business and invest in entities in federally-legal jurisdictions by including appropriate representations, warranties and covenants in its agreements with investees. However, an investee may breach such obligations. Any such violation of such obligation would result in a breach of the applicable agreement and, accordingly, may have a material adverse effect on the business, operations and financial condition of Company.
(xxvii) COVID-19
The current global uncertainty with respect to the spread of COVID-19, the rapidly evolving nature of the pandemic and local and international developments related thereto and its effect on the broader global economy and capital markets may impact the Company's business in the coming months.
The Company has taken proactive measures throughout the COVID-19 pandemic to protect the health and safety of its employees, to continue delivering high quality medical cannabis to its patients and to maintain its balance sheet.
While the precise impact of the COVID-19 outbreak on the Company remains unknown, rapid spread of COVID-19 and declaration of the outbreak as a global pandemic has resulted in travel advisories and restrictions, certain restrictions on business operations, social distancing precautions and restrictions on group gatherings which are having direct impacts on businesses in Canada, the State of Israel, Germany and around the world and could result in additional precautionary measures that could impact the Company's business. The spread of COVID-19 may also have a material adverse effect on global economic activity and could result in volatility and disruption to global supply chains and the financial and capital markets, which could interrupt supplies and other services from third parties upon which the Company relies, decrease demand for products, cause staff shortages, reduced customer traffic, and increased government regulation, all of which may materially and negatively impact the business, financial condition and results of operations of the Company.
(xxviii) Focus' Essential Service Designation
In response to the COVID-19 pandemic, the State of Israel has implemented mandatory shut-downs of non-essential businesses to prevent the spread of COVID-19. Focus' business has been deemed an "essential service", permitting it to continue production. There is no guarantee that further measures may nevertheless require Focus to shut down or limit its operations in the State of Israel. Any disruptions to the business and operations of Focus in the event that Focus were to lose its designation as an "essential service" in the State of Israel may materially and negatively impact the business, financial condition and results of operations of the Company.
|
IM Cannabis Corp. |
||
|
Management’s Discussion and Analysis |
(xxix) The Company's status as a "foreign private issuer" under U.S. securities laws
The Company is a "foreign private issuer", under applicable U.S. federal securities laws, and is, therefore, not subject to the same requirements that are imposed upon U.S. domestic issuers by the United States Securities and Exchange Commission (the "SEC"). Under the United States Exchange Act of 1934, as amended (the "Exchange Act"), the Company is subject to reporting obligations that, in certain respects, are less detailed and less frequent than those of U.S. domestic reporting companies. As a result, the Company does not file the same reports that a U.S. domestic issuer would file with the SEC, although the Company is required to file with or furnish to the SEC the continuous disclosure documents that it is required to file in Canada under Canadian securities laws. In addition, the Company's officers, directors, and principal shareholders are exempt from the reporting and short-swing profit recovery provisions of Section 16 of the Exchange Act. Therefore, the Company's shareholders may not know on as timely a basis when the Company's officers, directors and principal shareholders purchase or sell Common Shares, as the reporting periods under the corresponding Canadian insider reporting requirements are longer.
As a foreign private issuer, the Company is exempt from the rules and regulations under the Exchange Act related to the furnishing and content of proxy statements. The Company is also exempt from Regulation FD, which prohibits issuers from making selective disclosures of material non-public information. While the Company complies with the corresponding requirements relating to proxy statements and disclosure of material non-public information under Canadian securities laws, these requirements differ from those under the Exchange Act and Regulation FD and shareholders should not expect to receive the same information at the same time as such information is provided by U.S. domestic companies. In addition, the Company may not be required under the Exchange Act to file annual and quarterly reports with the SEC as promptly as U.S. domestic companies whose securities are registered under the Exchange Act.
In addition, as a foreign private issuer, the Company has the option to follow certain Canadian corporate governance practices, except to the extent that such laws would be contrary to U.S. securities laws, and provided that the Company disclose the requirements it is not following and describe the Canadian practices it follows instead. The Company may in the future elect to follow home country practices in Canada with regard to certain corporate governance matters. As a result, the Company's shareholders may not have the same protections afforded to shareholders of U.S. domestic companies that are subject to all corporate governance requirements.
(xxx) The Company may lose its status as a foreign private issuer under U.S. securities laws
In order to maintain its status as a foreign private issuer, a majority of the Company's Common Shares must be either directly or indirectly owned by non-residents of the U.S. unless the Company also satisfies one of the additional requirements necessary to preserve this status. The Company may in the future lose its foreign private issuer status if a majority of its Common Shares are held in the U.S. and if the Company fails to meet the additional requirements necessary to avoid loss of its foreign private issuer status. The regulatory and compliance costs under U.S. federal securities laws as a U.S. domestic issuer may be significantly more than the costs incurred as a Canadian foreign private issuer eligible to use the multi-jurisdictional disclosure system adopted by the securities regulatory authorities in United States and Canada ("MJDS"). If the Company is not a foreign private issuer, it would not be eligible to use the MJDS or other foreign issuer forms and would be required to file periodic and current reports and registration statements on U.S. domestic issuer forms with the SEC, which are more detailed and extensive than the forms available to a foreign private issuer. In addition, the Company may lose the ability to rely upon exemptions from NASDAQ corporate governance requirements that are available to foreign private issuers.
|
IM Cannabis Corp. |
||
|
Management’s Discussion and Analysis |
(xxxi) The Company's status as an "emerging growth company" under U.S. securities laws
The Company is an "emerging growth company" as defined in section 3(a) of the Exchange Act (as amended by the JOBS Act, enacted on April 5, 2012), and the Company will continue to qualify as an emerging growth company until the earliest to occur of: (a) the last day of the fiscal year during which the Company has total annual gross revenues of US$1,070,000,000 (as such amount is indexed for inflation every five years by the SEC) or more; (b) the last day of the fiscal year of the Company following the fifth anniversary of the date of the first sale of common equity securities of the Company pursuant to an effective registration statement under the U.S. Securities Act; (c) the date on which the Company has, during the previous three year period, issued more than US$1,000,000,000 in non-convertible debt; and (d) the date on which the Company is deemed to be a "large accelerated filer", as defined in Rule 12b-2 under the Exchange Act. The Company will qualify as a large accelerated filer (and would cease to be an emerging growth company) at such time when on the last business day of its second fiscal quarter of such year the aggregate worldwide market value of its common equity held by non-affiliates will be US$700,000,000 or more.
For so long as the Company remains an emerging growth company, it is permitted to and intends to rely upon exemptions from certain disclosure requirements that are applicable to other public companies that are not emerging growth companies. These exemptions include not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act. The Company cannot predict whether investors will find the Common Shares less attractive because the Company relies upon certain of these exemptions. If some investors find the Common Shares less attractive as a result, there may be a less active trading market for the Common Shares and the Common Share price may be more volatile. On the other hand, if the Company no longer qualifies as an emerging growth company, the Company would be required to divert additional management time and attention from the Company's development and other business activities and incur increased legal and financial costs to comply with the additional associated reporting requirements, which could negatively impact the Company's business, financial condition and results of operations.
Changes in Accounting Policies including Initial Adoption
The Company's significant accounting policies under IFRS are contained in the Annual Financial Statements (refer to Note 2 to the Annual Condensed Consolidated Financial Statements). Certain of these policies involve critical accounting estimates as they require management to make particularly subjective or complex judgments, estimates and assumptions about matters that are inherently uncertain and because of the likelihood that materially different amounts could be reported under different conditions or using different assumptions.
|
IM Cannabis Corp. |
||
|
Management’s Discussion and Analysis |
The following new accounting standards applied or adopted during the year ended December 31, 2020, had impact on the Annual Financial Statements:
IFRS 3, "Business Combinations":
In October 2018, the IASB issued an amendment to the definition of a "business" in IFRS 3, "Business Combinations" ("the Amendment"). The Amendment is intended to assist entities in determining whether a transaction should be accounted for as a business combination or as an acquisition of an asset.
The Amendment consists of the following:
1. Clarification that to meet the definition of a business, an integrated set of activities and assets must include, as a minimum, an input and a substantive process that together significantly contribute to the ability to create output.
2. Removal of the reference to the assessment whether market participants are capable of acquiring the business and continuing to operate it and produce outputs by integrating the business with their own inputs and processes.
3. Introduction of additional guidance and examples to assist entities in assessing whether the acquired processes are substantive.
4. Narrowing the definitions of "outputs" and "business" by focusing on goods and services provided to customers.
5. Introducing an optional concentration test that permits a simplified assessment of whether an acquired set of activities and assets is not a business.
The Amendment is to be applied prospectively to all business combinations and asset acquisitions for which the acquisition date is on or after the beginning of the first annual reporting period beginning on or after January 1, 2020, with earlier application permitted. The Amendment is not expected to have a material impact on the Company in the current or future reporting periods.
Amendment to IAS 1, "Presentation of Financial Statements":
In January 2020, the IASB issued an amendment to IAS 1, "Presentation of Financial Statements" ("the Amendment") regarding the criteria for determining the classification of liabilities as current or non-current.
The Amendment includes the following clarifications:
|
IM Cannabis Corp. |
||
|
Management’s Discussion and Analysis |
The Amendment is effective for annual periods beginning on or after January 1, 2023 and must be applied retrospectively.
The Company is evaluating the possible impact of the Amendment on its current loan agreements.
Financial Instruments
The Group has exposure to the following risks from its use of financial instruments:
Share price risk
The Group's investments in unlisted shares are sensitive to the market price risk arising from uncertainties about the future value of these investments. The Group manages the price risk through diversification and by placing limits on individual and total investment in shares.
The Company's board of directors reviews and approves all decisions related to investments in shares.
At the reporting date, the Group's exposure to investments in unlisted shares measured at fair value was $2,341.
Credit risk
The maximum credit exposure at December 31, 2020, is the carrying amount of cash and cash equivalents, accounts receivable and other current assets. The Group does not have significant credit risk with respect to customers. All cash and cash equivalents are placed with major Israeli financial institutions.
Liquidity risk
As at December 31, 2020, the Group's financial liabilities with liquidity risk consist of trade payables and other accounts payable, which have contractual maturity dates within one year, and lease liabilities. The Group manages its liquidity risk by reviewing its capital requirements on an ongoing basis. Based on the Group's working capital position as at December 31, 2020, management considers liquidity risk to be low. The table below summarizes the maturity profile of the Group's lease liabilities based on contractual undiscounted payments (including interest payments):
|
IM Cannabis Corp. |
||
|
Management’s Discussion and Analysis |
December 31, 2020:
|
|
Less than |
|
1 to 5 |
|
6 to 10 |
|
>10 |
|
|
|
|
|
|
|
|
|
|
Lease liabilities |
$ 232 |
|
$ 547 |
|
$ 515 |
|
$ - |
December 31, 2019:
|
|
Less than |
|
1 to 5 |
|
6 to 10 |
|
>10 |
|
|
|
|
|
|
|
|
|
|
Lease liabilities |
$ 229 |
|
$ 566 |
|
$ 553 |
|
$ 46 |
The maturity profile of the Company's other financial liabilities with liquidity risk (trade payables, other account payable and accrued expenses) as of December 31, 2020 and 2019, are less than one year.
Currency rate risk
As at December 31, 2020, a portion of the Group's financial assets and liabilities held in Euro and CAD consist of cash and cash equivalents in the amount of €472 (approximately $738) and $4,188, respectively. The Group's objective in managing its foreign currency risk is to minimize its net exposure to foreign currency cash flows by transacting, to the greatest extent possible, with third parties in NIS. The Group does not currently use foreign exchange contracts to hedge its exposure of its foreign currency cash flows, as management has determined that this risk is not significant at this point of time.
Procedures and Internal Control over Financial Reporting
Internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with applicable IFRS. Internal control over financial reporting should include those policies and procedures that establish the following:
• maintenance of records in reasonable detail, that accurately and fairly reflect the transactions and dispositions of assets;
• reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with applicable IFRS;
• receipts and expenditures are only being made in accordance with authorizations of management or the board of directors; and
|
IM Cannabis Corp. |
||
|
Management’s Discussion and Analysis |
• reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of the Company's assets that could have a material effect on the financial instruments.
The Company's management, with the participation of the Chief Executive Officer ("CEO") and Chief Financial Officer ("CFO"), assessed the effectiveness of the Company's internal controls over financial reporting and concluded that as at December 31, 2020, the Company's internal control over financial reporting was effective and yet constantly seek to improve it.
During the year ended December 31, 2020, the Company did not make any significant changes to its internal controls over financial reporting that would have materially affected, or reasonably likely to materially affect, its internal controls over financial reporting.
Limitations of Disclosure Controls and Procedures and Internal Control over Financial Reporting
The Company's management, including the CEO and CFO, believe that due to inherent limitations, any disclosure controls and procedures or internal control over financial reporting, no matter how well designed and operated, can provide only reasonable, not absolute, assurance of achieving the desired control objectives. Further, the design of a control system must reflect the fact that there are resource constraints, and the benefits of controls must be considered relative to their costs. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that any design will not succeed in achieving its stated goals under all potential future conditions. Accordingly, because of the inherent limitations in a cost- effective control system, misstatements due to error or fraud may occur and not be detected. Additionally, management is required to use judgment in evaluating controls and procedures.
Additional Information
Additional information relating to the Company, including the Company's AIF, is available on SEDAR at www.sedar.com.
- - - - - - - - - - - - - - - - - - - - - - - - -