Exhibit 99.3

Management’s Discussion and Analysis
|
TABLE OF CONTENTS
|
|
| EXECUTIVE SUMMARY | 4 |
| 6 |
|
| 6 |
|
| BRANDS | 8 |
| 12 |
|
| 12 |
|
| 13 |
|
| 18 |
|
| 44 |
|
| RISK FACTORS | 53 |
| CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS | 59 |
2
Management’s Discussion and Analysis
INTRODUCTION
IM Cannabis Corp. (“IM Cannabis” or the “Company”) is a British Columbia company operating in the international
medical cannabis industry. The Company’s common shares (the “Common Shares”) trade under the ticker symbol “IMCC” on both the NASDAQ Capital Market (“NASDAQ”) and the
Canadian Securities Exchange (“CSE”) as of March 1, 2021 and November 5, 2019, respectively.
This Management’s Discussion and Analysis (“MD&A”) reports on the consolidated financial condition and operating results of IM Cannabis for the three
months ended March 31, 2024. Throughout this MD&A, unless otherwise specified, references to “we”, “us”, “our” or similar terms, as well as the “Company” and “IM Cannabis” refer to IM Cannabis Corp., together with its subsidiaries, on a
consolidated basis, and the “Group” refers to the Company, its subsidiaries, and Focus Medical Herbs Ltd.
This MD&A should be read in conjunction with the interim condensed consolidated financial statements of the Company and the notes thereto for the three months ended March 31, 2024 (the "Interim Financial Statements") and with the Company's audited annual consolidated financial statements and the notes thereto for the years ended December 31, 2023 and 2022 (the “Annual
Financial Statements”). References herein to “Q1 2024” and “Q1 2023” refer to the three months ended March 31, 2024 and March 31, 2023, respectively, and references to “2023” refer to the year ended December 31, 2023.
The Interim Financial Statements have been prepared by management in accordance with the International Financial Reporting Standards (“IFRS”) as issued by
the International Accounting Standards Board (“IASB”). IFRS requires management to make certain judgments, estimates and assumptions that affect the reported amount of assets and liabilities at the date of
the Interim Financial Statements and the amount of revenue and expenses incurred during the reporting period. The results of operations for the periods reflected herein are not necessarily indicative of results that may be expected for future
periods. The Interim Financial Statements for the three months ended March 31, 2024, include the accounts of the Group, which includes, among others, the following entities:
|
Legal Entity
|
Jurisdiction
|
Relationship with the Company
|
|
I.M.C. Holdings Ltd. (“IMC Holdings”)
|
Israel
|
Wholly-owned subsidiary
|
|
I.M.C. Pharma Ltd. (“IMC Pharma”)
|
Israel
|
Wholly-owned subsidiary of IMC Holdings
|
|
I.M.C. Farms Israel Ltd. (“IMC Farms”)
|
Israel
|
Wholly-owned subsidiary of IMC Holdings
|
|
Focus Medical Herbs Ltd. (“Focus”)
|
Israel
|
Subsidiary of IMC Holdings *
|
|
R.A. Yarok Pharm Ltd. (“Pharm Yarok”)
|
Israel
|
Wholly-owned subsidiary of IMC Holdings
|
|
Rosen High Way Ltd. (“Rosen High Way”)
|
Israel
|
Wholly-owned subsidiary of IMC Holdings
|
|
Revoly Trading and Marketing Ltd. dba Vironna Pharm (“Vironna”)
|
Israel
|
Subsidiary of IMC Holdings
|
|
Oranim Plus Pharm Ltd. (“Oranim Plus”)
|
Israel
|
Subsidiary of IMC Holdings
|
|
Trichome Financial Corp. (“Trichome”)**
|
Canada
|
Wholly-owned subsidiary
|
* Effective February 26, 2024, IMC Holdings exercised its option to acquire a 74% ownership stake in Focus.
3
Management’s Discussion and Analysis
In this MD&A, unless otherwise indicated, all references: (i) “Company Subsidiaries” are to the Israeli Subsidiaries and Adjupharm, (ii) “Israeli Operations” are to IMC Holdings and the Israeli Subsidiaries and (iii) “Trichome” are to Trichome Financial Corp. and its subsidiaries.
All dollar figures in this MD&A are expressed in thousands of Canadian Dollars ($), except per share data and unless otherwise noted. All references to “NIS” are to New Israeli Shekels. All
references to “€” or to “Euros” are to Euros. All references to “US$” or to “U.S. Dollars” are to United States Dollars. The Company’s shares, options, units and warrants are not expressed in thousands. Prices are not expressed in thousands.
NON-IFRS FINANCIAL MEASURES
Certain non-IFRS financial measures are referenced in this MD&A that do not have any standardized meaning under IFRS, including “Gross Margin”, “EBITDA” and “Adjusted EBITDA”. The Company
believes that these non-IFRS financial measures and operational performance measures, in addition to conventional measures prepared in accordance with IFRS, enable readers to evaluate the Company’s operating results, underlying performance and
prospects in a similar manner to the Company’s management. For a reconciliation of these non-IFRS financial measures to the most comparable IFRS financial measures, as applicable, see the “Metrics and Non-IFRS
Financial Measures” section of the MD&A.
NOTE REGARDING THE COMPANY’S ACCOUNTING PRACTICES
The Company complies with IFRS 10 to consolidate the financial results of Focus, a holder of an Israeli Medical Cannabis Agency (the “IMCA”) license which
allows it to import and supply cannabis products, on the basis of which IMC Holdings exercises “de facto control”. For a full explanation of the Company’s application of IFRS 10, see “Legal and Regulatory –
Restructuring” and “Legal and Regulatory – Risk Factors”. As of February 26, 2024, IMC Holdings holds 74% of Focus shares.
OVERVIEW – CURRENT OPERATIONS IN ISRAEL AND GERMANY
IM Cannabis is an international cannabis company that is currently focused on providing premium cannabis products to medical patients in Israel and Germany, two of the world’s largest federally
legal cannabis markets. Until recently, the Company was also actively servicing adult-use recreational consumers in Canada, however the Company has exited operations in Canada and considers these operations discontinued. The Company leverages a
transnational ecosystem powered by a unique data-driven approach and a globally sourced product supply chain. With an unwavering commitment to responsible growth and compliance with the strictest regulatory environments, the Company strives to
amplify its commercial and brand power to become a global high-quality cannabis player.
On November 7, 2022, the Company has exited its operations in Canada, deconsolidated Trichome pursuant to IFRS10 and announced that it is pivoting its focus and resources to achieve sustainable and
profitable growth in its highest value markets, Israel and Germany, while also commencing its exit from the Canadian cannabis market. The Canadian operations was wound-down under the Canadian Companies’ Creditors Arrangement Act (“CCAA”) under the supervision of the Ontario Superior Court of Justice (Commercial List) (the “Court”) (the “CCAA Proceedings”). The
Company has exited operations in Canada Pursuant to an Order of the Court made on April 6, 2023, in which the Court approved a share purchase agreement, selling certain of the Trichome and certain of Trichome's wholly owned subsidiaries
(collectively, the "Trichome Group") to a party that is not related to the Company.
4
Management’s Discussion and Analysis
In the context of the deconsolidation of the Canadian operations, there are no remaining liabilities to the Company or any of its consolidated subsidiaries related to the Canadian entities, except
tax obligation of $839 related to debt settlement with L5 Capital Inc. (“L5 Capital”). The CCAA Proceedings were solely in respect of the Trichome Group. As such, the Company’s other assets or subsidiaries,
including those in Israel and Germany, were not parties to the CCAA Proceedings. Court materials filed in connection with Trichome's CCAA Proceedings can be found at: https://www.ksvadvisory.com/insolvency-cases/case/trichome.
In Israel, the Company imports, distributes and sells cannabis to local medical patients by operating medical cannabis retail pharmacies, online platforms, distribution center and logistical hubs
operating through IMC Holdings’ subsidiaries and Focus, leveraging proprietary data and patient insights. The Company also preserves its existing proprietary genetics with third-party cultures facilities in Israel.
In Germany, the IM Cannabis ecosystem operates through Adjupharm, importing and distributing cannabis to pharmacies for patients, and acting as the Company’s entry point for potential Europe-wide
distribution in the future.
With the recent regulatory changes in both Israel and in Germany, the market dynamics are changing.
Germany legalized cannabis on April 1, 2024, facilitating the access to medical cannabis prescriptions for patients and legalizing non-profit social clubs starting July 1, 2024. The change in
regulation has already led to rapid expansion within the first month, driven by the number of new patients entering into the market, highlighting the importance of a stable supply chain able to respond quickly to increases in demand.
The proposed Israeli medical cannabis regulatory reform entered into vigor on April 1, 2024, as well. The reform is also expected to facilitate the access to medical cannabis for many new patient
groups. While the impact in Germany was reflected immediately in the market, the Israeli reform is starting slowly and will take time for the impact to be reflected in the market.
For further information regarding the Germany new legislation and the Israeli Reform, please see sections "Regulatory Framework in Israel" and " Regulatory Framework in Germany" below.
OUR GOAL – DRIVE PROFITABLE REVENUE GROWTH
Our primary goal is to sustainably increase revenue in each of our core markets, to accelerate our path to profitability and long-term shareholder value while actively managing costs and margins.
HOW WE PLAN TO ACHIEVE OUR GOAL – CORE STRATEGIES
Our strategy of sustainable and profitable growth consists of:
| • |
Continue building on the increasing demand and positive momentum in Israel and Germany, supported by strategic alliances with Canadian suppliers and a highly skilled sourcing team, to cement its leadership position in markets where the
Company operates.
|
| • |
Develop and execute a long-term growth plan in Germany, based on the strong sourcing infrastructure in Israel which is powered by advanced product knowledge and regulatory expertise establishing, in the Company’s view, a competitive
advantage following the April 1, 2024 legalization in Germany.
|
| • |
Properly position brands with respect to target-market, price, potency and quality, such as our IMC brand in Israel and Germany.
|
| • |
Strong focus on efficiencies and synergies as a global organization with domestic expertise in Israel and Germany.
|
| • |
High-quality, reliable supply to our customers and patients, leading to recurring sales.
|
| • |
Ongoing introduction of new Stock Keeping Unit (“SKUs”) to keep consumers and patients engaged.
|
5
Management’s Discussion and Analysis
RESULTS – REVENUE GROWTH IN Q1 2024
The Company operates in the Israeli and German medical cannabis markets. The Company was also actively servicing adult-use recreational consumers in Canada, however these operations were
discontinued and deconsolidated, effective November 7, 2022, pursuant to IFRS10. The Company announced that it is pivoting its focus and resources to achieve sustainable and profitable growth in its highest value markets, Israel, Germany and Europe
implementing a leaner organization strategy with the primary focus on achieving profitability in 2024.
Israel
In Israel, we continue to expand IMC brand recognition and supply the growing Israeli medical cannabis market with our branded products. The Company offers medical cannabis patients a rich variety
of high-end medical cannabis products through strategic alliances with Canadian suppliers supported by a highly skilled sourcing team. In addition to the benefits of the Group’s long-term presence in Israel, we believe that with our strong sourcing
infrastructure in Israel, and advanced product knowledge, regulatory expertise and strong commercial partnerships, the Company is well-positioned to address the ongoing needs and preferences of medical cannabis patients in Israel.
The Company is also operating in the retail segment. The Company, through IMC Holdings, holds three licensed pharmacies, each selling medical cannabis products to patients: (i) Oranim Plus, a
pharmacy in Jerusalem Israel, (ii) Vironna, a leading pharmacy in the Arab sector, and (iii) Pharm Yarok, the largest pharmacy in the Sharon plain area and the biggest call center in the country (Oranim Plus, Vironna, and Pharm Yarok collectively,
the “Israeli Pharmacies”).
On April 16, 2024, the Company announced that following a reconciliation between the parties regarding all remaining unpaid installments (i.e. NIS 5,363K or 1,930K CAD) by IMC Holdings, relating to
the Oranim Pharmacy Acquisition completed on March 28, 2022, the parties have mutually agreed to revoke the transaction. As a result, IMC Holdings Ltd. shares (51%) will be transferred back to the seller.
6
Management’s Discussion and Analysis
The Company has also acquired home-delivery services and an online retail footprint, operating under the name “Panaxia-to-the-Home”, which includes a
customer service center and an Israeli medical cannabis distribution licensed center (the “Panaxia Transaction”), from Panaxia Pharmaceutical Industries Israel Ltd. and Panaxia Logistics Ltd., part of the
Panaxia Labs Israel, Ltd. group of companies (collectively, “Panaxia”). On June 30, 2023, IMC Pharma, the entity responsible for operating the trading house that was acquired within the Panaxia Transaction,
ceased its operations at the aforementioned licensed center located in Lod, Israel. Consequently, the Company transitioned the operation that was conducted through IMC Pharma to third-party entities and to its own trading house currently being
operated by Rosen High Way and there are no material obligations remained open following closure of the trading house.
The operation in the retail segment in Israel positions IM Cannabis as a large distributor of medical cannabis in Israel. We are strategically focused on establishing and reinforcing a direct
connection with medical cannabis patients, providing direct access to IM Cannabis products, obtaining and leveraging market data and gaining a deeper understanding of consumer preferences. The operation of the Israeli Pharmacies allows the Company
to increase purchasing power with third-party product suppliers, offers potential synergies with our established call center and online operations, achieves higher margins on direct sales to patient and creates the opportunity for up-sales across a
growing range of products.
Germany
In Europe, the Company operates in Germany through Adjupharm, its German subsidiary and EU-GMP certified medical cannabis producer and distributor. We continue to lay our foundation in Germany,
which is currently the European market with the largest number of medical cannabis patients.1 Leveraging our global supply
chain, IM Cannabis continues to focus on growing its business in Germany to be well-positioned through brand recognition in preparation for future regulatory reforms.
Similar to Israel, the Company’s focus in Germany is to import dried cannabis from its supply partners, which we believe will satisfy the rapid growth in demand for high-THC cannabis across a
variety of strains and qualities. In addition, Adjupharm sells cannabis extracts to meet the existing demand in the German market.
In the Company’s view, the strong sourcing infrastructure in Israel, powered by advanced product knowledge and regulatory expertise, will establish a competitive advantage in Germany after the
April 1, 2024, legalization.. This is based on the premise that the German and Israeli markets share a number of common attributes such as robust commercial infrastructure, highly developed digital capabilities, favourable demographics and customer
preferences.
While the Company does not currently distribute products in other European countries, the Company intends to leverage the foundation established by Adjupharm, its state-of-the-art warehouse and
EU-GMP production facility in Germany (the “Logistics Center”), its vast knowledge in the cannabis market and costumers’ preferences and its network of distribution partners to expand into other jurisdictions
across the continent.
Adjupharm has an EU-GMP license that permits it to engage in additional production, cannabis testing and release activities. It allows Adjupharm to repackage bulk cannabis, to perform stability
studies and offer such services to third parties.
1 The European Cannabis Report – Edition 7 https://prohibitionpartners.com/2022/03/31/launching-today-the-european-cannabis-report-7th-edition/
and Visual Capitalist website, A Bird’s Eye View of the World’s Largest Cannabis Markets
https://www.visualcapitalist.com/sp/a-birds-eye-view-of-the-worlds-largest-cannabis-markets/
7
Management’s Discussion and Analysis
The IMC brand is well-known in the Israeli medical cannabis market, with reputable brands highly popular among Israeli consumers.
Israeli Medical Cannabis Business
The IMC brand has established its reputation in Israel for quality and consistency over the past 10 years and more recently with new high-end, ultra-premium strains that have made it to the
top-sellers list in pharmacies across the country.
The Group maintains a portfolio of strains sold under the IMC umbrella from which popular medical cannabis dried flowers and full-spectrum cannabis extracts are produced.
The IMC brand offers four different product lines, leading with the Craft Collection which offers the highest quality Canadian craft cannabis flower and has established IMC as the leader of the
super-premium segment in Israel.
The Craft Collection – The IMC brand’s premium product line with indoor-grown, hand-dried and hand-trimmed high-THC cannabis flowers. The Craft Collection
includes exotic and unique cannabis strains such as Cherry Crasher, Wedding Crasher, Peanut Butter MAC and Watermelon Zkittlez. During the year ended December 31, 2023, the Company was selling products under the Craft Collection, however, In Q4 the
Craft Collection was temporarily unavailable for sale by the Company and as a result, the Company did not generate any sales under the Craft Collection brand during that time. During Q1 2024 the Company relaunched sales under the Craft Collection.

|
The Top-Shelf Collection – IMC’s premium product line, which offers indoor-grown, high-THC cannabis flowers with strains
such as Lemon Rocket, Diesel Drift, Tropicana Gold, Lucy Dreamz, Santa Cruz, Or'enoz and Banjo. Inspired by the 1970’s cannabis culture in America, the Top-Shelf Collection targets the growing segment of medical patients who are cannabis
culture enthusiasts.
|
 |
8
Management’s Discussion and Analysis


The Signature Collection – The IMC brand’s high-quality product line with greenhouse-grown or indoor grown, high-THC cannabis flowers. The Signature
Collection currently includes well known proprietary cannabis dried flowers such as Roma® Chemchew, Karmalada, Rockabye, Silver haze, all an indoor-grown flowers.


The Full Spectrum Extracts – The IMC brand’s full spectrum, strain-specific cannabis extracts, includes high-THC Roma®T20 oil.
As part of its recent rebranding the Company expanded its Roma® product portfolio to include also oils. IMC’s Roma® strain is a high-THC medical cannabis
flower that offers a therapeutic continuum and is known for its strength and longevity of effect.
9
Management’s Discussion and Analysis
| The WAGNERS™ brand launched in Israel in Q1 2022, with indoor-grown cannabis imported from Canada. The WAGNERS™ brand was the first international premium, indoor-grown brand introduced to the Israel cannabis market, at a competitive price point. The WAGNERS™ brand includes Cherry Jam re-launched in Q3 2023, Pink Bubba, Golden Ghost, Tiki Rain, Rain, Forest Crunch and Silverback#4. |
 |

|
BLKMKT™, the Company’s second Canadian brand, super-premium product line with indoor-grown, hand-dried and hand-trimmed high-THC cannabis flowers.
The BLKMKT™ includes JEALOUSY, BACIO GLTO, PNPL P, PARK FIRE OG, UPSIDE DOWN C. In Q4 2023 the Company re-launched JEALOUSY and BACIO GLTO.
|


10
Management’s Discussion and Analysis
|
LOT420 brand launched in Israel in Q2 2023, with super-premium indoor-grown
cannabis imported from Canada with high-THC. The LOT420 includes ICY C, GLTO 33, Atomic APP and as of Q3 2023 also Glto 33 and Xeno. The Company ceased from
selling Atomic APP.
|
 |


The PICO collection (minis)- Under the BLKMKT™ and LOT420 brands, the Company launched in 2023 a new type of products (small flowers), a super-premium
indoor-grown cannabis imported from Canada with high-THC. In Q4 2023 the Company re-launched PICO JEALOUSY #1 and PICO BACIO GLTO #4 T20i. In addition, the Company launched in Q4 2023 a new cannabis strain called Pico Upside Down #05 T20H.

For more information, see “Strategy in Detail – Brands – New Product Offerings” section of the MD&A.
11
Management’s Discussion and Analysis
German Medical Cannabis Business
In Germany, the company sells IMC-branded dried flower products and full spectrum extracts. The medical cannabis products are branded generically as IMC to increase the brand awareness and build
brand heritage among the German healthcare professionals.
After launching the first high THC strain in 2020, the portfolio has been carefully curated to include 5 high THC flowers, 1 high CBD flower and 3 full spectrum extracts with the goal of providing
German physicians and patients with a more complete portfolio.
Since 2023, the company has been number one in sales per SKU within the entire German flower market. The company is the sixth largest cannabis company in Germany. The Group’s competitive advantage
in Germany lies in its track record, experience and brand reputation in Israel, as well as the proprietary data supporting the potential effectiveness of medical cannabis for the treatment of a variety of conditions.

Between our various geographies, the strategy for new products varies given that each market is at a different stage of development with respect to regulatory regimes, patient and customer
preferences and adoption rates.
Israel
In Q1 2024, the Company launched new cannabis strains in Israel, namely "PURPLE RAIN T 15", "YA HEMI" and "BACIO GELATO" by BLKMKT™, "SUPER SATIVA" by TENZO
AVANT, "GELATO 33" by LOT420, "MOTORBRTH", "B F LMO" and "FLO OG" by SNDL.
Israel
The company is concentrating on leveraging its skilled sourcing team and strategic alliances with Canadian suppliers as well as the import of medical cannabis from its Canadian Facilities. The
Company continues to import cannabis products and supply medical cannabis to patients through licensed pharmacies. To supplement growing demand, the Company continue its relationships with third-party cultivation facilities in Israel for the
propagation and cultivation of the Company’s existing proprietary genetics and for the development of new products.
12
Management’s Discussion and Analysis
In addition, the Company is operating through its subsidiaries who obtained a license from the IMCA to, among others, import cannabis products and supply medical cannabis to patients.
Pursuant to the applicable Israeli cannabis regulations, following the import of medical cannabis, medical cannabis products are then packaged by contracted GMP licensed producers of medical
cannabis. The packaged medical cannabis products are then sold by the Group under the Company’s brands to local Israeli pharmacies directly or through contracted distributors.
Germany
The Company continues to expand its presence in the German market by forging partnerships with pharmacies and distributors across the country and developing Adjupharm and its Logistics Center as
the Company’s European hub. Adjupharm sources its supply of medical cannabis for the German market and from various EU-GMP certified European and Canadian suppliers. The Logistics Center is EU-GMP certified, upgrading Adjupharm’s production
technology and increasing its storage capacity to accommodate its anticipated growth. Adjupharm has a certification for primary repackaging, making it one of a handful of companies in Germany fully licenced to repack bulk.
Adjupharm currently holds wholesale, narcotics handling, manufacturing, procurement, storage, distribution, and import/export licenses granted to it by the applicable German regulatory authorities
(the “Adjupharm Licenses”).
KEY HIGHLIGHTS FOR THE FIRST QUARTER OF 2024
In the first quarter of 2024, the Company continued to focus on its efforts and resources on growth in the Israeli and German cannabis markets with a goal of reaching profitability during 2024. The
Company’s key highlights and events for the first quarter ended March 31, 2024, include:
Loan from ADI
On October 11, 2022, IMC Holdings entered into a loan agreement with A.D.I. Car Alarms Stereo Systems Ltd (“ADI” and the “ADI Agreement”),
to borrow a principal amount of NIS 10,500 thousand (approximately $4 million) at an annual interest of 15% (the “ADI Loan”), which is to be repaid within 12 months of the date of the ADI Agreement. The ADI
Loan is secured by a second rank land charge on the Logistics Center of Adjupharm. In addition, CEO and Director of the Company, provided a personal guarantee to ADI should the security not be sufficient to cover the repayment of the ADI Loan.
On October 25, 2023, IMC Holdings and ADI signed an amendment to the ADI Agreement, extending the loan period by an additional 3 months. During this extended period, the interest rate will be 15%,
with associated fees and commissions of 3% per annum for the application fee and an origination fee of 3% per annum.
13
Management’s Discussion and Analysis
On February 26, 2024, IMC Holdings and ADI signed an additional amendment to the ADI Agreement, extending the loan period until April 15, 2024, with the same terms as the first amendment, as
specified above.
IMC Holdings and ADI are negotiating a repayment plan of the ADI Loan.
Acquisition of Jerusalem’s Leading Medical Cannabis Pharmacy – Oranim Pharm
On January 12, 2024, the Company announced that the final sixth payment of the Oranim Pharmacy Acquisition and the reconciliation between the parties regarding the remaining transaction payments
are being rescheduled to April 15, 2024.
Through the transaction, completed on March 28, 2022, IMC Holdings Ltd. acquired 51% of the rights in Oranim Pharm Partnership through the acquisition of Oranim Plus. As part of the transaction
consideration, NIS 5,363K or 1,930K CAD were supposed to be paid in six installments throughout 2023, with the final payment due February 15, 2024.
Through a new amendment signed January 10, 2024, the sixth (6) payment as well as the reconciliation between the parties regarding all remaining unpaid installments has been postponed to April 15,
2024. All six installments (that remain unpaid) will incur a 15% interest charge. Failure to meet the remaining payments will result in the transfer of IMC Holdings Ltd. shares (51%) back to the seller, along with the revocation of the transaction.
On April 16, 2024, the Company announced that further to the news release dated January 12, 2024, the Company has decided not to make remaining installment payments installments (i.e. NIS
5,873K including interest or 2,154K CAD) by IMC Holdings, and as such will transfer the 51% shares held by IMC Holdings back to the seller.
NASDAQ Compliance Notice
On January 31, 2024, the Company received an extension a 180-calendar day extension, until July 29, 2024, from Nasdaq staff to regain compliance with the Minimum Share Price Listing Requirements
(the “Extension”). As at the date of this MD&A, the Company continues to monitor the closing bid price of its Common Shares and plans to pursue available options to regain compliance with the Minimum
Share Price Listing Requirement, including potentially pursuing a reverse stock split. If the Company authorizes a reverse stock split, it will plan to effectuate the split no later than ten business days prior to the end of the Extension.
Potential Reverse Merger with Kadimastem
On February 28, 2024, the Company announced that it has entered into a non-binding term sheet dated February 13, 2024, as amended (the “Term Sheet”), and a
Loan Agreement (as defined below) with Holding Company (as defined below), with Israel-based Kadimastem Ltd a clinical cell therapy public company traded on the Tel Aviv Stock Exchange under the symbol (TASE:KDST) (“Kadimastem”), whereby the parties will complete a business combination that will constitute a reverse merger into the Company by Kadimastem (the “Proposed Transaction”).
The Proposed Transaction will be effected by way of a plan of arrangement involving a newly created wholly-owned subsidiary of IMC and Kadimastem (the “Arrangement”).
The resulting issuer that will exist upon completion of the Proposed Transaction (the “Resulting Issuer”) will change its business from medical cannabis to biotechnology and, at the closing of the Proposed
Transactions (the "Closing"), Kadimastem shareholders will hold 88% of the common shares of the Resulting Issuer (the “Resulting Issuer Shares”) and the shareholders of the Company will hold 12% of the
Resulting Issuer Share. Parties may agree, in the Definitive Agreement, on a different structure of equity in lieu of the warrants (as described below) with a similar result. The Proposed Transaction is an arm’s length transaction.
14
Management’s Discussion and Analysis
Prior to Closing, IMC's existing medical cannabis operation and other current activities in Israel and Germany (the "Legacy Business") will be restructured
(the “Spin-Out”) as a contingent value right (the "CVR"). The CVR will entitle the holders thereof to receive net cash, equity, or other net value upon the sale of the
Legacy Business following the Closing, subject to the terms of the Loan Agreement.
To facilitate the sale of the Legacy Business, a special committee of IMC's Board was formed, which will oversee the potential sale in collaboration with legal and financial advisors.
The Legacy Business will be made available for potential sale to a third party for a period of up to 12 months from Closing (the "Record Date"). After the
Record Date, any remaining Legacy Business in the CVR will be offered for sale through a tender process, subject to the terms of the best offer. The proceeds from the sale of the Legacy Business will be utilized to settle debts and distribute the
remaining balance, if any, to CVR holders.
As a condition of Closing, Kadimastem will have approximately $5 million in gross funds, at Closing including capital raised concurrently with the completion of the Proposed Transaction from
existing shareholders and additional investors.
In addition to the foregoing, subject to compliance with applicable law, the Company shall grant shareholders of the Company as of Closing, with warrant(s) equal their pro rata portion, of 2% of
the Resulting Issuer’s issued and outstanding common share capital (the "IMC Shares") prior to the Closing Date (in the aggregate), with an exercise price per share equal to the 10 day volume-weighted average
price of the Resulting Issuer’s shares calculated on the NASDAQ Capital Market (“Nasdaq”), ending 2 trading days prior to Closing, the warrants will be for a period of 24 months following Closing.
In accordance with the terms of the Proposed Transaction, the holders of the issued and outstanding shares in the capital of Kadimastem (the “Kadimastem Shares”)
will be issued such number of IMC Shares in exchange for every one (1) Kadimastem Share held immediately prior to the completion of the Proposed Transaction that reflects the ratio outlined above (the “Exchange
Ratio”). Outstanding convertible securities of Kadimastem (the “Kadimastem Convertible Securities”) will be treated through customary mechanics as shall be determined in the definitive agreement,
which may include, the assumption of the Kadimastem Convertible Securities by IMC subject to customary adjustments to reflect the Exchange Ratio and exercise price.
Pursuant to the terms of the Term Sheet, a loan agreement dated February 28, 2024 (the "Loan Agreement") was entered between IMC Holdings Ltd. a wholly-owned
subsidiary of IMC (the "Holding Company") and Kadimastem. Pursuant to the Loan Agreement, Kadimastem will provide a loan of up to US$650,000 to the Holding Company, funded in two installments: US$300,000 upon
signing the Loan Agreement and US$350,000 upon the execution of the definitive agreement regarding the Proposed Transaction (the "Loan").
15
Management’s Discussion and Analysis
The Loan accrues interest at a rate of 9.00% per annum, compounding annually and is secured by the following collaterals and guarantees: (a) 10% of the proceeds derived from any operation sale
under the CVR (“Charged Rights”), limited to the outstanding Loan Amount and expenses according to the Loan Agreement, accordingly Holding Company may, at its sole discretion, to record a second-ranked fixed charge over the Charged Rights or,
alternatively, in case the existing pledges over the Charged Rights at the date of signing this Loan Agreement are subsequently discharged or removed, then the Borrower shall promptly record a first-ranking fixed charge over the Charged Assets with
all applicable public records; provided that Holding Company shall not impose any new lien, mortgage, charge or pledge over the Charged Rights that did not exist on the date hereof, or any other liens, subject to customary exclusions; (b) the
Holding Company shall use its best efforts to record a first-ranking fixed charge over the assets of its subsidiary, A.R Yarok Pharm Ltd, in due course when applicable and as deemed appropriate; and (c) a personal guarantee by Mr. Oren Shuster,
IMC’s CEO.
Prior to the completion of the Proposed Transaction, IMC will call a meeting of its shareholders for the purpose of approving, among other matters:
| • |
approve the Proposed Transaction;
|
| • |
approve the Spin-Out;
|
| • |
a change of name of the Company as directed by Kadimastem and acceptable to the applicable regulatory authorities effective upon Closing; and
|
| • |
reconstitution of the Company’s Board.
|
Upon closing of the Proposed Transaction, all of IMC’s current directors and executive officers will resign and the board of directors of the Resulting Issuer will, subject to the approval of
governing regulatory bodies, consist of nominees of Kadimastem. All of the executive officers shall be replaced by nominees of Kadimastem, all in a manner that complies with the requirements of governing regulatory bodies and applicable securities
and corporate laws.
Details of insiders and proposed directors and officers of the Resulting Issuer will be disclosed in a further news release.
The completion of the Proposed Transaction is subject to a number of conditions, including but not limited to the following:
| • |
the execution of a definitive agreement;
|
| • |
completion of mutually satisfactory due diligence;
|
| • |
completion of the Share Consolidation; and
|
| • |
receipt of all required regulatory, corporate and third party approvals, including approvals by governing regulatory bodies, the shareholders of IMC and Kadimastem, applicable Israeli governmental authorities, and the fulfilment of all
applicable regulatory requirements and conditions necessary to complete the Proposed Transaction.
|
The parties are committed to seeking a successful completion of the Proposed Transaction as soon as practicable, but there can be no absolute certainty that the Proposed Transaction will take place.
Option to re-acquire the sold interest in Focus
On November 30, 2023, IMC Holdings acted to exercise its option to purchase the 74% interest in Focus held by Oren Shuster and Rafael Gabay by submitting a request to the IMCA which approved the transaction on February
25, 2024. As of February 26, 2024, IMC Holdings holds 74% of Focus shares.
16
Management’s Discussion and Analysis
SUBSEQUENT EVENTS
The new Israeli cannabis regulation
On April 1, 2024, the Company announced the implementation of the medical cannabis regulatory reform in Israel starting as of April 1, 2024.
The reform, announced by the Israeli ministry of health on August 7, 2023, underwent a three-month delay due to the Israel-Hamas war following its initial announcement (the "Reform").
The Reform will be implemented in phases, as approved, and announced by the Israeli Ministry of Health. The key aspects of the initial phase, commencing today, April 1st, are as follows:
1. Change in the prescription process: patients with a wide range of diseases and medical conditions from Oncology to Parkinsons will no longer be required to obtain a license to receive medical
cannabis. Patients will receive a prescription similar to those for other prescription medications. Pain and PTSD are not included in the Reform yet.
2. Medical cannabis will now be prescribed through the HMO's, Israel's public healthcare system: until the Reform, cannabis could not be prescribed through the HMO's which cover the majority of the
Israeli population.
3. The number of prescribing physicians is expected to increase: as of today, HMO physicians, who are dully trained and certified within their field of expertise, can prescribe medical cannabis as
a first line treatment, as opposed to a last resort, based on medical discretion for the approved indications. 4. The cost for prescription is anticipated to be reduced: the Ministry of Health limited the cost for a medical cannabis prescription.
For the full report published by the Ministry of Health see (in Hebrew)- https://www.health.gov.il/hozer/mmk152_2016.pdf
Trademark Licensing Agreement
On April 4, 2024, the Company and Avant Brands Inc. (TSX: AVNT) (OTCQX: AVTBF) (FRA: 1BU0) ("Avant") a leading producer of innovative cannabis products,
jointly announce the signing of an international trademark licensing agreement (the "Licensing Agreement") granting Adjupharm the exclusive right to launch the BLK MKT™ brand in the German medical cannabis
market. The Licensing Agreement constitutes another major milestone with respect to the relationship between the two cannabis companies.
Under the terms of the Trademark License Agreement, Avant's subsidiary will grant Adjupharm the license to utilize Avant's BLK MKT TM cannabis brand for use on their medical cannabis products. All
such products will contain cannabis cultivated exclusively by Avant and subsequently exported to Germany. The collaboration between the two companies anticipates a positive outcome in the emerging German medical cannabis market, especially
following the recent legalization by the government on April 1, 2024.
The Licensing Agreement signals the Company's commitment to implementing a premium strategy in Germany as well as in Israel and acts as another step to establish Avant's position in the
ultra-premium segment in Israel and Germany. The Company and Avant have had a productive partnership so far, combining Avant's premium cannabis products with the Company's sales, marketing and distribution expertise in Israel. Both companies
believe the Licensing Agreement will enhance the companies' capabilities to meet the demands of the German market.
Avant's three largest cultivation facilities all hold ICANN-GAP and GACP certifications; thus, Avant is positioned to potentially distribute its premium cannabis flower into international markets.
Adjupharm is the 6th largest distributor of medical cannabis flowers in Germany and is number 1 in sales per SKU, growing +180% in 20232.
2 Insight Health December 2023
17
Management’s Discussion and Analysis
Partnership with Flora Growth
On April 9, 2024, the Company announced that it has entered, through its subsidiary, a strategic distribution agreement with Vessel Brand Inc, a subsidiary of Flora Growth Corp. ("Flora"), a global consumer-packaged goods leader and pharmaceutical distributor, headquartered in Carlsbad, CA.
The Company's brands are well known in the premium Israeli cannabis market, facilitating the import and wholesale of premium medical cannabis through retail pharmacies, online platform, and
distribution center. Vessel is a premium cannabis accessories brand with a wide range of products.
Short-term Loan Agreement
On October 17, 2023, IMC Holdings entered into a short-term loan agreement with a non-financial institute in the amount of NIS 1,800 thousand (approximately $610). Such loan bear interest at an
annual rate of 18% and mature six months from the date of issuance along with the associated fees and commissions of 4% per annum for application fee and an origination fee of 4% per annum. On April 17, 2024, IMC Holdings and the lender signed an
amendment to extend the loan period until April 18, 2025, with an annual interest rate of 17% with no additional fees associated as in the initial loan period.
Loan Agreement
On April 17, 2024 R.A Yarok Pharm entered into a loan agreement with a non-financial institute in the amount of NIS 3,000 thousand (approximately $1,082). Such loan bear interest at an annual rate
of 15% and mature 12 months from the date of issuance (the "Loan"). The Loan is secured by the following collaterals and guarantees: (a) a first-ranking floating charge over the assets of A.R Yarok Pharm (b)
a first-ranking fixed charge over the holdings (23.3%) of its subsidiary, IMC Holdings, of Xinteza; (c) a personal guarantee by Mr. Oren Shuster, IMC’s CEO and (D) a guarantee by the Company.
FINANCIAL HIGHLIGHTS
Below is the analysis of the changes that occurred for the three months ended March 31, 2024, with further commentary provided below.
18
Management’s Discussion and Analysis
|
For the three months
ended March 31
|
For the year ended
December 31
|
|||||||||||
|
2024
|
2023(1)
|
2023
|
||||||||||
|
Net Revenues
|
$
|
12,063
|
$
|
12,529
|
$
|
48,804
|
||||||
|
Gross profit before fair value impacts in cost of sales
|
$
|
1,789
|
$
|
3,243
|
$
|
10,830
|
||||||
|
Gross margin before fair value impacts in cost of sales (%)
|
15
|
%
|
26
|
%
|
22
|
%
|
||||||
|
Operating Loss
|
$
|
(5,630
|
)
|
$
|
(3,617
|
)
|
$
|
(12,792
|
)
|
|||
|
Net loss
|
$
|
(6,020
|
)
|
$
|
(866
|
)
|
$
|
(10,228
|
)
|
|||
|
Loss per share attributable to equity holders of the Company – Basic (in CAD)
|
$
|
(0.42
|
)
|
$
|
(0.05
|
)
|
$
|
(0.74
|
)
|
|||
|
Loss per share attributable to equity holders of the Company - Diluted (in CAD)
|
$
|
(0.42
|
)
|
$
|
(0.05
|
)
|
$
|
(0.74
|
)
|
|||
|
For the three months
ended March 31
|
For the year ended
December 31
|
|||||||||||
|
2024
|
2023
|
2023
|
||||||||||
|
Average net selling price of dried flower (per Gram)
|
$
|
5.68
|
$
|
6.59
|
$
|
5.14
|
||||||
|
Quantity of dried flower sold (in Kilograms)
|
1,873
|
1,842
|
8,609
|
|||||||||
Notes:
|
1.
|
The figures disclosed here for the three months ended March 31, 2023, encompass updates and adjustments made during Q2 2023 to the Company’s previously filed unaudited interim financial statements. The
adjustments and updates were immaterial. .
|
The Overview of Financial Performance includes reference to “Gross Margin”, which is a non-IFRS financial measure that the Company defines as the difference between revenue and cost of revenues
divided by revenue (expressed as a percentage), prior to the effect of a fair value adjustment for inventory and biological assets. For more information on non-IFRS financial measures, see the “Non-IFRS Financial
Measures” and “Metrics and Non-IFRS Financial Measures” sections of the MD&A.
OPERATIONAL RESULTS
In each of the markets in which the Company operates, the Company must navigate evolving customer and patient trends in order to continue to be competitive with other suppliers of medical cannabis
products.
The Company believes that there are several key factors creating tailwinds to facilitate further industry growth. In Israel, the number of licensed medical patients currently stands at 135,071 as
of March 2024. This figure is expected to grow in the coming years and may further benefit from regulatory change liberalizing the cannabis market in Israel IM Cannabis is a large distributor of medical cannabis in Israel. As the Israeli cannabis
market has become increasingly competitive, the ability to import premium cannabis from Canada is a key determinant of the Company’s success in Israel.
The German medical cannabis market has been slower over the past few years to develop mainly due to the difficulty in medical patients accessing prescriptions and insurance reimbursements. Starting
Year 2024 after the legalization was officially approved by the Bundestag (Germany Parliament) on March 1st 2024, The Company which has already seen an increase in the
number of patients paying out-of-pocket for medical cannabis products in Germany during the past few years, is expecting a change which will lead to increase in the market. Starting April 2024, the Company through its subsidiary Adjupharm is
experiencing a significant increase in demands in Germany for its products.
19
Management’s Discussion and Analysis
REVENUES AND GROSS MARGINS
REVENUES
The revenues of the Group are primarily generated from sales of medical cannabis products to customers in Israel and Germany. The reportable geographical segments in which the Company operates are Israel and Germany.
For the three months ended March 31:
|
Israel
|
Germany
|
Adjustments
|
Total
|
|||||||||||||||||||||||||||||
|
2024
|
2023(*)
|
2024
|
2023(*)
|
2024
|
2023(*)
|
2024
|
2023(*)
|
|||||||||||||||||||||||||
|
Revenues
|
$
|
10,911
|
$
|
11,437
|
$
|
1,152
|
$
|
1,092
|
$
|
-
|
$
|
-
|
$
|
12,063
|
$
|
12,529
|
||||||||||||||||
|
Segment loss
|
$
|
(4,508
|
)
|
$
|
(1,618
|
)
|
$
|
(316
|
)
|
$
|
(557
|
)
|
$
|
-
|
$
|
-
|
$
|
(4,824
|
)
|
$
|
(2,175
|
)
|
||||||||||
|
Unallocated corporate expenses
|
$
|
-
|
$
|
-
|
$
|
-
|
$
|
-
|
$
|
(806
|
)
|
$
|
(1,442
|
)
|
$
|
(806
|
)
|
$
|
(1,442
|
)
|
||||||||||||
|
Total operating (loss)
|
$
|
(4,508
|
)
|
$
|
(1,618
|
)
|
$
|
(316
|
)
|
$
|
(557
|
)
|
$
|
(806
|
)
|
$
|
(1,442
|
)
|
$
|
(5,630
|
)
|
$
|
(3,617
|
)
|
||||||||
|
Depreciation, amortization
|
$
|
655
|
$
|
780
|
$
|
25
|
$
|
29
|
$
|
-
|
$
|
-
|
$
|
680
|
$
|
809
|
||||||||||||||||
* See Note 1 under “Review of Financial Performance – Financial Highlights” section of the MD&A.
The consolidated revenues of the Group for the three months ended March 31, 2024, were attributed to the sale of medical cannabis products in Israel and Germany.
| ● |
Revenues for the three months ended March 31, 2024 and 2023 were $12,063 and $12,529, respectively, representing a decrease of $466 or 4%. The decrease is mainly due to Exchange rate effect of about $0.2 and decrease in Avg. price per
sale due to increased competition.
|
| ● |
Revenues from the Israeli operation were attributed to the sale of medical cannabis through the Company’s agreement with Focus Medical and the revenues from the Israeli Pharmacies the Company owns, mostly from cannabis products.
|
| ● |
In Germany, Company revenues were attributed to the sale of medical cannabis through Adjupharm.
|
| ● |
Total dried flower sold for the three months ended March 31, 2024, was 1,873kg at an average selling price of $5.68 per gram compared to 1,842kg for the same period in 2023 at an average selling price of
$6.59 per gram, mainly attributed to the inventory life cycle, discounts given and increased competition in the segment.
|
| ● |
Cost of Revenues
|
Cost of revenues is comprised of purchase of raw materials and finished goods, import costs, production costs, product laboratory testing, shipping and salary expenses. Inventory
is later expensed to the cost of sales when sold. Direct production costs are expensed through the cost of sales.
The cost of revenues for the three months ended March 31, 2024 and 2023 were $10,274 and $9,286, respectively, representing an increase of $988 or 11%. This is mainly due
to clearing of old inventory of approximately $140K and accrued for slow inventory of approximately $500K.
20
Management’s Discussion and Analysis
GROSS PROFIT
Gross profit for the three months ended March 31, 2024, and 2023 was $1,779 and $2,904, respectively, representing a decrease of $1,125 or 39%.
Gross profit included losses from unrealized changes in fair value of biological assets and realized fair value adjustments on inventory sold of $(10) and $(339) for the three months ended March
31, 2024, and 2023, respectively.
EXPENSES
GENERAL AND ADMINISTRATIVE
General and administrative expenses for the three months ended March 31, 2024, and 2023 were $2,332 and $3,175, respectively, representing a decrease of $843 or 27%.
The decrease in the general and administrative expense is attributable mainly to the restructuring plan published March 8, 2023, which aimed for reorganization of the company's management and
operations to strengthen its focus on core activities and drive efficiencies to realize sustainable profitability. The Company reduced its workforce in Israel across all functions. The general and administrative expenses are comprised mainly from
salaries to employees in the amount of $572, professional fees in the amount of $699, depreciation and amortization in the amount of $121 and insurance costs in the amount of $326.
PROVISION FOR REVOKING ORANIM TRANSACTION
Due to the revocation of the Oranim agreement on April 16, 2024, the Company recorded $2,753 other operating expenses for losses expected on Q2 2024 due to the clearing of Oranim assets and
liabilities from the consolidated balances. The total expense for the 3 months ended March 31, 2024 is $2,753.
SELLING AND MARKETING
Selling and marketing expenses for the three months ended March 31, 2024, and 2023 were $2,292 and $2,805, respectively, representing a decrease of $513 or 18%. The decrease in the selling and marketing expenses was
due mainly to decrease salaries to employees of $429 due to the saving plan executed in H2 2023.
SHARE-BASED COMPENSATION
Share-based compensation expense for the three months ended March 31, 2024, and 2023 was $32 and $258, respectively, representing a decrease $226 or 88%. The decrease was mainly attributed to the cancellation of
incentive stock options (“Options”) held by employees who no longer worked for the Company.
FINANCING
Financing income (expense) net for the three months ended March 31, 2024, and 2023 was $(501) and $2,735, respectively, representing a decrease of $3,236 or 118% in the financing income.
21
Management’s Discussion and Analysis
NET INCOME/LOSS
Net loss for the three months ended March 31, 2024, and 2023 was $6,020 and $866, respectively, representing a net loss decrease of $5,154 or 595%. The net loss decrease related to factors impacting net income
described above.
NET INCOME (LOSS) PER SHARE BASIC AND DILUTED
Basic loss per share is calculated by dividing the net profit attributable to holders of Common Shares by the weighted average number of Common Shares outstanding during the period. Diluted profit per Common Share is
calculated by adjusting the earnings and number of Common Shares for the effects of dilutive warrants and other potentially dilutive securities. The weighted average number of Common Shares used as the denominator in calculating diluted profit
per Common Share excludes unissued Common Shares related to Options as they are antidilutive. Basic and diluted Income (Loss) per Common Share for the three months ended March 31, 2024, and 2023 were $(0.42) and $(0.05) per Common Share,
respectively.
TOTAL ASSETS
Total assets as at March 31, 2024 were $41,109, compared to $48,813 as at December 31, 2023, representing a decrease of $7,704 or 16%. This decrease was primarily due to the reduction of cash and cash equivalents in
the amount of $765 and inventory reduction of $2,075, decrease of $1,145 in trade receivables and $2,653 in Goodwill.
TOTAL LIABILITIES
Total liabilities at March 31, 2024, were $32,765, compared to $35,113 at December 31, 2023, representing a decrease of $2,348 or 7%. The decrease was mainly due to a decrease in PUT option
liability in the amount of $730, a decrease in advances from customers in the amount of $711 and a decrease in accrued expenses of $558.
LIQUIDITY AND CAPITAL RESOURCES
For the three months ended March 31, 2024, the Company recorded revenues of $12,063.
The Company can face liquidity fluctuations from time to time, resulting from delays in sales and slow inventory movements.
In January 2022, Focus entered into a revolving credit facility with an Israeli bank, Bank Mizrahi (the “Mizrahi Facility”). The Mizrahi Facility is
guaranteed by Focus assets. Advances from the Mizrahi Facility will be used for working capital needs. The Mizrahi Facility has a total commitment of up to NIS 15 million (approximately $6,000) and has a one-year term for on-going needs and 6
months term for imports and purchases needs. The Mizrahi Facility is renewable upon mutual agreement by the parties. The borrowing base is available for draw at any time throughout the Mizrahi Facility and is subject to several covenants to be
measured on a quarterly basis (the “Mizrahi Facility Covenants”).
The Mizrahi Facility bears interest at the Israeli Prime interest rate plus 1.5%.
On May 17, 2023, the Company and Bank Mizrahi entered to new credit facility with total commitment of up to NIS 10,000 (approximately $3,600) (the “New Mizrahi
Facility”). The New Mizrahi Facility consists of NIS 5,000 credit line and NIS 5,000 loan to be settled with 24 monthly installments from May 17, 2023. This loan bears interest at the Israeli Prime interest rate plus 2.9%. As of Marcg 31,
2024 Focus has drawn down $3,111 in respect of the new Mizrahi facility (comprised of approx. $1,845 credit line and $1,266 loan). The New Credit facility is also subject to several covenants to be measured on a quarterly basis which are not met as
of March 31, 2024, therefore the loan is classified as short-term loan.
22
Management’s Discussion and Analysis
The Company's CEO and director, provided to the bank a personal guarantee in the amount of the outstanding borrowed amount, allowing the New Mizrahi Facility to remain effective.
As of March 31, 2024, the Group's cash and cash equivalents totaled $1,048 and the Group's working capital deficit (current assets less current liabilities) amounted to ($10,518). In the three months ended March 31,
2024, the Group had an operating loss of ($5,630) and negative cash flows from continuing operating activities of ($662).
As of March 31, 2024, the Group’s financial liabilities consisted of accounts payable which have contractual maturity dates within one year. The Group manages its liquidity risk by reviewing its
capital requirements on an ongoing basis. Based on the Group’s working capital position on March 31, 2024, management considers liquidity risk to be high.
As of March 31, 2024, the Group has identified the following liquidity risks related to financial liabilities (undiscounted):
|
Less than one year
|
1 to 5 years
|
6 to 10 years
|
> 10 years
|
|||||||||||||
|
Contractual Obligations
|
$
|
12,446
|
$
|
1,222
|
-
|
-
|
||||||||||
The maturity profile of the Company’s other financial liabilities (trade payables, other account payable and accrued expenses, and warrants) as of March 31, 2024, are less than one year.
|
Payments Due by Period
|
||||||||||||||||||||
|
Contractual Obligations
|
Total
|
Less than one year
|
1 to 3 years
|
4 to 5 years
|
After 5 years
|
|||||||||||||||
|
Debt
|
$
|
12,342
|
$
|
11,941
|
$
|
401
|
$
|
-
|
$
|
-
|
||||||||||
|
Finance Lease Obligations
|
$
|
1,326
|
$
|
505
|
$
|
778
|
$
|
43
|
$
|
-
|
||||||||||
|
Total Contractual Obligations
|
$
|
13,668
|
$
|
12,446
|
$
|
1,179
|
$
|
43
|
$
|
-
|
||||||||||
The Group’s current operating budget includes various assumptions concerning the level and timing of cash receipts from sales and cash outflows for operating expenses and capital expenditures, including cost saving
plans. In 2023 The Company’s board of directors approved a cost saving plan, to allow the Company to continue its operations and meet its cash obligations. The cost saving plan entailed reducing costs through efficiencies and synergies primarily
involving the following measures: discontinuing loss-making activities, reducing payroll and headcount, reduction in compensation paid to key management personnel (including layoffs of key executives), operational efficiencies and reduced capital
expenditures. These actions are expected to result in cost savings in 2024 and the company will continue its efforts for efficiency operations.
Despite the cost savings plan and restructuring as described above, the projected cash flows for 2024 indicates that it is uncertain that the Group will generate sufficient funds to continue its
operations and meet its obligations as they become due. The Group continues to evaluate additional sources of capital and financing. However, there is no assurance that additional capital and or financing will be available to the Group, and even if
available, whether it will be on terms acceptable to the Group or in amounts required.
23
Management’s Discussion and Analysis
These conditions raise substantial doubt about the Company’s ability to continue as a going concern. The consolidated financial statements do not include any adjustments relating to the
recoverability and classification of assets or liabilities that might be necessary should the Company be unable to continue as a going concern. The Interim Financial Statements have been prepared on the basis of accounting principles applicable to
a going concern, which assumes that the Company will continue in operation for the foreseeable future and will be able to realize its assets and discharge its liabilities in the normal course of operations. The Interim Financial Statements do not
include any adjustments to the amounts and classification of assets and liabilities that would be necessary should the Company be unable to continue as a going concern. Such adjustments could be material.
SHARE CAPITAL
The Company’s authorized share capital consists of an unlimited number of Common Shares without par value 13,394,136 of which were issued and outstanding as at the date hereof. The Common Shares
confer upon their holders the right to participate in the general meeting with each Common Share carrying the right to one vote on all matters. The Common Shares also allow holders to receive dividends if and when declared and to participate in the
distribution of surplus assets in the case of liquidation of the Company.
OTHER SECURITIES
As of March 31, 2024, the Company also has the following outstanding securities which are convertible into, or exercisable or exchangeable for, voting or equity securities of the Company: 270,452
Options, 18,261 2019 Broker Compensation Options (as defined below), 294,348 Offered Warrants (as defined below) and 5,769,611 2023 LIFE Offering Warrants.
FINANCIAL BACKGROUND
On October 11, 2019, the Company completed the Reverse Takeover Transaction, effected by way of a “triangular merger” between the Company, IMC Holdings and a wholly owned subsidiary of the Company
pursuant to Israeli statutory law.
In connection with the Reverse Takeover Transaction, the Company completed a private placement offering of 19,460,527 subscription receipts (each a “Subscription
Receipt”) on a pre-2021 Share Consolidation basis (as defined below) of a wholly owned subsidiary of the Company at a price of $1.05 per Subscription Receipt for aggregate gross proceeds of $20,433. Upon completion of the Reverse Takeover
Transaction, each Subscription Receipt was exchanged for one unit comprised of one (1) common share and one-half of one (1/2) warrant (each whole warrant, a “2019 Listed Warrant”). Each 2019 Listed Warrant
was exercisable for one Common Share at an exercise price of $1.30 until October 11, 2021. A total of 9,730,258 2019 Listed Warrants were issued and listed for trading on the CSE under the ticker “IMCC.WT”. The 2019 Listed Warrants expired on
October 11, 2021.
The Company also issued to the agent who acted on its behalf in connection with the Reverse Takeover Transaction, a total of 1,199,326 2019 Broker Compensation Options (the “2019 Broker Compensation Options”). Following the 2021 Share Consolidation, the 2019 Broker Compensation Options were adjusted to require four 2019 Broker Compensation Options to be exercised for one underlying
unit at an adjusted exercise price of $4.20, with each unit exercisable into one Common Share and one-half of one Common Share purchase warrant (the “2019 Unlisted Warrants”). Following the 2021 Share
Consolidation, the 2019 Unlisted Warrants were adjusted to require four 2019 Unlisted Warrants to be exercised for one Common Share at an adjusted exercise price of $5.20. The 2019 Broker Compensation Options and the 2019 Unlisted Warrants expired
on August 2022.
24
Management’s Discussion and Analysis
On February 12, 2021, the Company consolidated all of its issued and outstanding Common Shares on the basis of one (1) post-consolidation Common Share for each four (4) pre-consolidation Common
Shares (the “2021 Share Consolidation”) to meet the NASDAQ minimum share price requirement.
On November 17, 2022, the Company completed a second share consolidation (the “2022 Share Consolidation”) by consolidating all its issued and outstanding
Common Shares on the basis of one (1) post-Consolidation Common Share for each ten (10) pre-Consolidation Common Shares.
On May 7, 2021, the Company completed an offering (the “2021 Offering”) for a total of 6,086,956 Common Shares and 3,043,478 Common Share purchase warrants
(the “2021 Offered Warrants”). Following the 2022 Share Consolidation, the 2021 Offered Warrant were adjusted to require the (10) 2021 Offered Warrant to be exercised for one (1) Common Share at an adjusted
exercise price of US$72 for a term of 5 years from the date of closing of the 2021 Offering.
The Company also issued a total of 182,609 broker compensation options (the “2021 Broker Compensation Options”) to the agents who acted on its behalf in
connection with the 2021 Offering. Following the 2022 Share Consolidation, the 2021 Broker Compensation Option were adjusted to require the (10) 2021 Broker Compensation Options for one (1) Common Share at an adjusted exercise price of US$66.1, at
any time following November 5, 2021 until November 5, 2024. There are 182,609 2021 Broker Compensation Options outstanding.
As of March 31, 2024, and December 31, 2023, there were 6,063,960 and 6,063,960 warrants outstanding, respectively, re-measured by the Company, using the Black-Scholes pricing model, in the amount of $137 and $38,
respectively. For the three months ended March 31, 2024, and 2023, the Company recognized a revaluation gain (loss) in the consolidated statement of profit or loss and other comprehensive income, of $(99) and $3,371, respectively, in which the
unrealized gain is included in finance income (expense).
25
Management’s Discussion and Analysis
OPERATING, FINANCING AND INVESTING ACTIVITIES
The following table highlights the Company’s cash flow activities for the three months ended March 31, 2024 and 2023:
|
For the three months ended March 31,
|
For the year ended December 31,
|
|||||||||||
|
Net cash provided by (used in):
|
2024
|
2023
|
2023
|
|||||||||
|
Operating activities
|
$
|
(662
|
)
|
$
|
(6,061
|
)
|
$
|
(8,075
|
)
|
|||
|
Investing activities
|
$
|
(2
|
)
|
$
|
(467
|
)
|
$
|
(1,182
|
)
|
|||
|
Financing activities
|
$
|
(852
|
)
|
$
|
6,557
|
$
|
9,417
|
|||||
|
Effect of foreign exchange
|
$
|
751
|
$
|
(1,059
|
)
|
$
|
(796
|
)
|
||||
|
Increase (Decrease) in cash
|
$
|
(765
|
)
|
$
|
(1,030
|
)
|
$
|
(636
|
)
|
|||
Operating activities used cash of $662 and $6,061 for the three months ended March 31, 2024, and 2023, respectively. This variance is primarily due to business activities of the Company including corporate expenses
for salaries, professional fees, and marketing expenses in Israel and, Germany.
Investing activities used cash of $2 and $467 for the three months ended March 31, 2024, and 2023, respectively. Decrease derived mainly from a decrease in purchase of property, plant and equipment in the amount of
$409.
Financing activities used cash of $852 and provided cash of $6,557 for the three months ended March 31, 2024, and 2023, respectively. This decrease is primarily due to the reduction of proceeds from issuance of
warrants and share capital by $7,203 and $649 respectively and set-off by an increase in proceeds from discounted checks in the amount of $2,581 and an increase of repayment of bank loan and credit facilities in the amount of $1,810.
SELECTED INTERIM INFORMATION – CONTINUING OPERATIONS
|
For the year ended
|
December 31,
2023 |
December 31,
2022
|
December 31,
2021
|
|||||||||
|
Revenues
|
$
|
48,804
|
$
|
54,335
|
$
|
34,053
|
||||||
|
Net Loss
|
$
|
(10,228
|
)
|
$
|
(24,922
|
)
|
$
|
(664
|
)
|
|||
|
Basic net income (Loss) per share:
|
$
|
(0.74
|
)
|
$
|
(3.13
|
)
|
$
|
0.02
|
||||
|
Diluted net income (Loss) per share:
|
$
|
(0.74
|
)
|
$
|
(3.81
|
)
|
$
|
(3.62
|
)
|
|||
|
Total assets
|
$
|
48,813
|
$
|
60,676
|
$
|
129,066
|
||||||
|
Total non-current liabilities
|
$
|
2,305
|
$
|
3,060
|
$
|
21,354
|
||||||
26
Management’s Discussion and Analysis
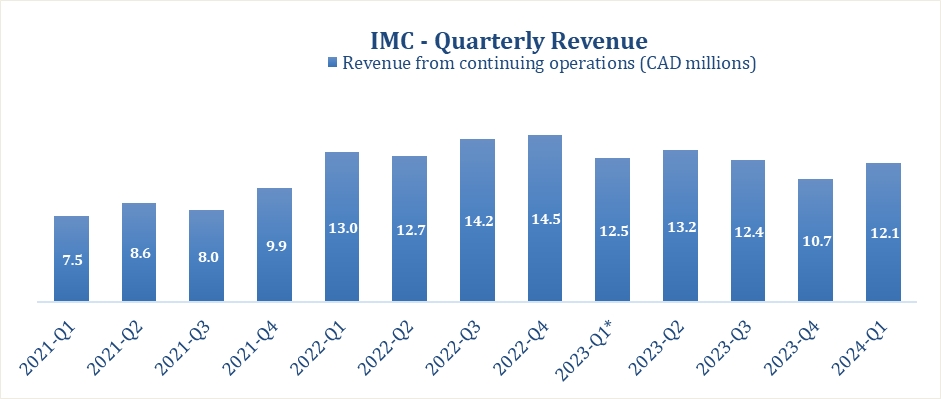
SUMMARY OF QUARTERLY RESULTS
|
For the three months ended
|
March 31, 2024
|
December 31, 2023
|
September 30, 2023
|
June 30, 2023
|
||||||||||||
|
Revenues
|
$
|
12,063
|
$
|
10,698
|
$
|
12,370
|
$
|
13,207
|
||||||||
|
Net Loss
|
$
|
(6,020
|
)
|
$
|
(3,520
|
)
|
$
|
(2,136
|
)
|
$ |
(3,706
|
)
|
||||
|
Basic net income (Loss) per share:
|
$
|
(0.42
|
)
|
$
|
(0.25
|
)
|
$
|
(0.16
|
)
|
$
|
(0.26
|
)
|
||||
|
Diluted net loss per share:
|
$
|
(0.42
|
)
|
$
|
(0.25
|
)
|
$
|
(0.16
|
)
|
$
|
(0.26
|
)
|
||||
|
For the three months ended
|
March 31, 2023 (1)
|
December 31, 2022
|
September 30, 2022
|
June 30, 2022
|
||||||||||||
|
Revenues
|
$
|
12,529
|
$
|
14,461
|
$
|
14,170
|
$
|
12,703
|
||||||||
|
Net income (Loss)
|
$
|
(866
|
)
|
$
|
(9,650
|
)
|
$
|
(4,532
|
)
|
$
|
(3,736
|
)
|
||||
|
Basic net income (Loss) per share:
|
$
|
(0.05
|
)
|
$
|
(1.32
|
)
|
$
|
(0.06
|
)
|
$
|
(0.27
|
)
|
||||
|
Diluted net income (Loss) per share:
|
$
|
(0.05
|
)
|
$
|
(1.28
|
)
|
$
|
(0.06
|
)
|
$
|
(0.30
|
)
|
||||
Note 1 - The figures disclosed here for the three months ended March 31, 2023, encompass updates and adjustments made during Q2 2023 to the Company’s previously filed unaudited interim financial
statements. The adjustments and updates were immaterial..
METRICS AND NON-IFRS FINANCIAL MEASURES
This MD&A makes reference to “Gross Margin”, “EBITDA”, and “Adjusted EBITDA”. These financial measures are not recognized measures under IFRS and do not have a standardized meaning prescribed
by IFRS and are therefore unlikely to be comparable to similar measures presented by other companies. Rather, these measures are provided as additional information to complement those IFRS measures by providing further understanding of our results
of operations from management’s perspective. Accordingly, these measures should neither be considered in isolation nor as a substitute for analysis of our financial information reported under IFRS.
Management defines Gross Margin as the difference between revenue and cost of goods sold divided by revenue (expressed as a percentage), prior to the effect of a fair value adjustment for inventory
and biological assets. Management defines EBITDA as income earned or lost from operations, as reported, before interest, tax, depreciation and amortization. Adjusted EBITDA is defined as EBITDA, adjusted by removing other non-recurring or non-cash
items, including the unrealized change in fair value of biological assets, realized fair value adjustments on inventory sold in the period, share-based compensation expenses, and revaluation adjustments of financial assets and liabilities measured
on a fair value basis. Management believes that Adjusted EBITDA is a useful financial metric to assess its operating performance on a cash adjusted basis before the impact of non-recurring or non-cash items. The closest IFRS metric to EBITDA and
Adjusted EBITDA is “operating loss”.
27
Management’s Discussion and Analysis
The non-IFRS financial measures can provide investors with supplemental measures of our operating performance and thus highlight trends in our core business that may not otherwise be apparent when
relying solely on IFRS measures. We also believe that securities analysts, investors and other interested parties frequently use non-IFRS financial measures in the evaluation of issuers. These financial measures are metrics that have been adjusted
from the IFRS statements in an effort to provide readers with a normalized metric in making comparisons more meaningful across the cannabis industry. However, other companies in our industry may calculate this measure differently, limiting their
usefulness as comparative measures.
Our management also uses these non-IFRS financial measures in order to facilitate operating performance comparisons from period to period, to prepare annual operating budgets and forecasts and to
determine components of management compensation. As required by Canadian securities laws, we reconcile these non-IFRS financial measures to the most comparable IFRS measures.
GROSS MARGIN
|
Three months ended
|
March 31, 2024
|
March 31, 2023(*)
|
||||||
|
Net Revenue
|
$
|
12,063
|
$
|
12,529
|
||||
|
Cost of sales
|
$
|
10,274
|
$
|
9,286
|
||||
|
Gross profit before FV adjustments
|
$
|
1,789
|
$
|
3,243
|
||||
|
Gross margin before FV adjustments (Non-IFRS)
|
15
|
%
|
26
|
%
|
||||
* See Note 1 under “Review of Financial Performance – Financial Highlights” section of the MD&A.
28
Management’s Discussion and Analysis
EBITDA AND ADJUSTED EBITDA
|
For the three months
ended March 31, |
For the year ended December 31,
|
|||||||||||
|
2024
|
2023(*)
|
2023
|
||||||||||
|
Operating Loss
|
$
|
(5,630
|
)
|
$
|
(3,617
|
)
|
$
|
(12,792
|
)
|
|||
|
Add: Depreciation & Amortization
|
$
|
680
|
$
|
809
|
$
|
2,996
|
||||||
|
EBITDA (Non-IFRS)
|
$
|
(4,950
|
)
|
$
|
(2,808
|
)
|
$
|
(9,796
|
)
|
|||
|
Add: IFRS Biological assets fair value adjustments, net (1)
|
$
|
10
|
$
|
339
|
$
|
984
|
||||||
|
Add: Share-based payments
|
$
|
32
|
$
|
258
|
$
|
225
|
||||||
|
Add: Restructuring cost (2)
|
$
|
-
|
$
|
283
|
$
|
617
|
||||||
|
Add: Other non-recurring costs (3)
|
$
|
2,753
|
$
|
-
|
$
|
-
|
||||||
|
Adjusted EBITDA (Non-IFRS)
|
$
|
(2,155
|
)
|
$
|
(1,928
|
)
|
$
|
(7,970
|
)
|
|||
* See Note 1 under “Review of Financial Performance – Financial Highlights” section of the MD&A.
Notes:
| 1. |
Losses from unrealized changes in fair value of biological assets and realized fair value adjustments on inventory. See “Cost of Revenues” section of the MD&A.
|
| 2. |
Costs attributable to the Israel Restructuring and closure of Sde Avraham Farm in 2022, and to Israel reorganization plan of the company’s management and operations in 2023.
|
| 3. |
Due to revocation of the Oranim transaction dated April 16, 2024.
|
The Company’s Adjusted EBITDA loss for the three months ended March 31, 2024, was increased, primarily due to, savings in the Company’s general and administrative expenses such as insurance cost reduction, cost
efficiencies from synergies and other corporate expenses reduction.
CONTINGENT LIABILITIES AND COMMITMENTS
RENTAL LIABILITIES
The table below summarizes the maturity profile of the Group’s lease liabilities based on contractual undiscounted payments (including interest payments):
March 31, 2024:
|
Less than one year
|
1 to 5 years
|
6 to 10 years
|
>10 years
|
|||||||||||||
|
Lease liabilities
|
$
|
505
|
$
|
821
|
-
|
-
|
||||||||||
December 31, 2023:
|
Less than one year
|
1 to 5 years
|
6 to 10 years
|
>10 years
|
|||||||||||||
|
Lease liabilities
|
$
|
1,886
|
-
|
-
|
-
|
|||||||||||
29
Management’s Discussion and Analysis
LITIGATION AND REGULATORY PROCEEDINGS
PLANNING AND CONSTRUCTION 66813-06-21 BEER SHEVA MAGISTRATE COURT
On July 11, 2021, the Company was informed that on June 30, 2021, a claim was filed to Beer Sheva Magistrate Court, by the municipal committee presiding over planning and construction in southern
Israel against Focus, Focus’ directors and officers, including Oren Shuster and Rafael Gabay, and certain landowners, claiming for inadequate permitting for construction relating to the Focus Facility (the “Construction
Proceedings”).
On December 6, 2021 the defendants filed a motion request for dismissal the indictment on the ground of defense of justice. The municipal committee filed its response and after that the defendants
filed a response to the municipal committee’s response. As of the date of this letter no decision has yet been made on the application.
A hearing was initially set to December 1, 2021, but postponed several times in order to allow the parties to negotiate towards a resolution. The hearing was set to June 22, 2023. A draft agreement
between the parties sent by the defendant to the municipal committee in order for it to be sent to the state attorney’s office for their comments, which once obtained, will be filed with the Court for its approval. The Court is not obligated to
approve the agreement between the parties, if obtained.
On June 22, 2023, a hearing took place before the esteemed Honorable Judge Orit Kertz. During the hearing it was decided that the defendants and the municipal committee's attorney would engage in
negotiations and make diligent efforts to reach a settlement before August 15, 2023. The responsibility of informing the court about any progress concerning a potential settlement was assigned to the attorney representing the municipal committee.
On September 9, 2023, the municipal committee's attorney was summoned to appear at a hearing before the Honorable Judge Orit Kertz. The hearing was postponed to December 28, 2023 due to the 2023 Israel–Hamas war as will be further specified under
“RISK FACTORS"” section of the MD&A.
On January 2, 2024, the Company announced that the construction proceedings against Focus, were concluded on December 28, 2023. The company maintains "de facto" control of Focus. Focus was indicted
and a fine of CAD$129,000 has been imposed. The cultivation facility, which was the focus of the proceedings, was closed in June 2022 in alignment with the Company's strategic shift towards import and sales.
COVID-19 TEST KITS CLAIM, DISTRICT COURT OF STUTTGART
On November 19, 2021, Adjupharm filed a statement of claim (the “Claim”) to the District Court of Stuttgart (the “Stuttgart Court”) against Stroakmont & Atton Trading GmbH (“Stroakmont & Atton”), its shareholders and managing directors regarding a debt owed by Stroakmont & Atton to
Adjupharm in an amount of approximately EUR 947,563 for COVID-19 test kits purchased by Stroakmont & Atton from Adjupharm at the end of March 2021. In January 2022, Stroakmont & Atton filed its statement of defence to the Stuttgart Court in
which they mainly stated two arguments for their defense:
| 1. |
The contractual party of the Company was not the Stroakmont & Atton. The contract with Stroakmont & Atton was only concluded as a sham transaction in order to cover up a contract with a company named Uniclaro GmbH. Therefore,
Stroakmont & Atton is not the real purchaser rather than Uniclaro GmbH.
|
| 2. |
The Company allegedly placed an order with Uniclaro GmbH for a total of 4.3 million Clongene COVID-19 tests, of which Uniclaro GmbH claims to have a payment claim against the Company for a partial delivery of 380,400 Clongene COVID-19
tests in the total amount of EUR 941,897.20. Uniclaro GmbH has assigned this alleged claim against the Company to Stroakmont & Atton Trading GmbH, and Stroakmont & Atton Trading GmbH has precautionary declared a set-off against the
Company’s claim.
|
30
Management’s Discussion and Analysis
On March 22, 2022, Adjupharm filed a response to Stroakmont & Atton’s statement of defence and rejected both allegations with a variety of legal arguments and facts and also offered evidence to
the contrary in the form of testimony from the witnesses in question.
The burden of proof for the allegation that the contract with Stroakmont & Atton was only concluded as a sham transaction lies with the opponents and they offered evidences to the court in the
form of testimony from certain witnesses.
A court hearing with witnesses was held on January 11, 2023 and on February 22, 2023, where witnesses testified.
According to the court the witnesses were not able to provide required evidence for the allegation regarding the sham transaction with Stroakmont & Atton. On April 5, 2023, Stuttgart Court
announced its decision (the "Judgment") and sentenced Stroakmont & Atton to pay to Adjupharm EUR 947,563.68 plus interest in the amount of 5 percentage points above the German basis rate since May 8,
2021. In addition, Stroakmont & Atton was sentences to pay Adjupharm EUR 6,551.20 plus interest at 5 percentage points above the German basis rate since December 14, 2021.
The directors of Stroakmont, Mr. Simic and Mr. Lapeschi, were not sentenced and in this respect, the claim was dismissed against them with regard to their personal liability. Adjupharm shall pay
2/3 of the Stuttgart Court expenses and the out-of-court expenses of Mr. Simic and Mr. Lapeschi. Stroakmont shall bear 1/3 of the Stuttgart Court expenses and 1/3 of the out-of-court expenses of Adjupharm. The remaining out-of-court expenses shall
be borne by each party.
Furthermore, the court did not decide on the counterclaims from an alleged order by Adjupharm for 4.3 million Clongene tests due to a set-off prohibition. This set-off prohibition follows from a
jurisdiction agreement concluded between Adjupharm and Uniclaro, which determined the courts in Hamburg to be the competent court to decide about such allegations.
The Judgment is not yet final and, therefore, cannot be enforced. On May 5, 2023, Adjupharm and Stroakmont & Atton, each submitted an appeal with the Stuttgart Court (the "Appeals") against the Judgment.
On June 23, 2023, Adjupharm filed its statement of grounds for appeal with the Higher Regional Court of Stuttgart. Adjupharm appeals against the fact that the directors of Stroakmont & Atton
were not sentenced to pay jointly and severally together with Stroakmont & Atton as a result of fraud. Since they concluded the purchase agreement with Adjupharm in the name of Stroakmont & Atton and there is indication that they did not
intend to pay the purchase price from the very beginning, this could be considered to be fraudulent inducement, for which they would be personally liable.
Stroakmont & Atton appeals against the judgement and request to reject the payment claim. Furthermore, they appeal against the prohibition of the set-off. Stroakmont & Atton are of the
opinion that there is no such prohibition, and they want to include their alleged counterclaims in the proceedings and to receive a decision for their counterclaim by the court in Stuttgart.
So far, there have not been any instructions from the Court of Appeal and no oral hearing was held yet. It is not yet possible to estimate the outcome of the appeal proceedings at this early stage
in the process.
At this stage, the Company management cannot assess its ability to collect the payment awarded in the Judgment and the chances of the claim advancing or the potential outcome of the Appeal.
31
Management’s Discussion and Analysis
UNICLARO GMBH VS. ADJUPHARM
On December 22, 2022, Uniclaro GmbH filed a statement of claim against Adjupharm with the district court in Hamburg. According to the statement of claim, Uniclaro GmbH is (“Uniclaro”) claiming the payment of the amount of EUR 1,046,010 (including VAT) in exchange for 300,000 Covid-19 rapid.
Uniclaro alleges in this lawsuit that Adjupharm purchased 4.3 million Covid-19 rapid tests of the brand "Clongene" from Uniclaro. Furthermore, Uniclaro claims that the order was placed verbally on
23.03.2021 and that Adjupharm has already paid for a portion of these tests and received them, but not yet the entire 4.3 million tests. They reserve the right to extend the lawsuit for a further amount (which they did not specify).
According to Uniclaro's statement of claim the lawsuit does not concern the same purchase price and the same Covid-19 rapid tests as in the Stroakmont & Atton Claim mentioned above. On 23
February 2023, the Company provided its statement of defense to the court. The statement of defense contains similar arguments to reject the allegations in this respect as in the court proceedings in Stuttgart about the counterclaims. Adjupharm
rejected the claim stating that it did not purchase such an amount if Covid-19 rapid tests, but only small portions on a case-by-case-basis and according to the available cash flow.
On February 14, 2024, a court hearing took place before the district court of Hamburg, at which the court also took evidence. The court first heard the managing directors of Uniclaro and Adjupharm. They commented on
the events of March 23, 2021 and the alleged purchase. The statements of all managing directors differed from each other. Afterwards, the witness Francesco Bisceglia, who holds the position of Sales Director at Adjupharm, was also heard. His
statement also partially deviated from the statements of all managing directors, but overall, the witness basically testified that the company did not purchase 4.3 million Clungene Tests in the meeting of 23. March 2021. The transcript of the
hearing is still pending.
The court provided the parties a deadline until March 27, 2024, to evaluate the statements of the managing directors and the testimony of the witness and to deliver to the court a summary of the
factual and legal situation after the court hearing.
On April 24, 2024 the Regional Court of Hamburg announced its decision. The judgment is as follows:
| 1. |
Adjupharm was not sentenced. Uniclaro's lawsuit for payment of EUR 1,046,010.00 in exchange for delivery of 300,000 Clungene tests was dismissed.
|
| 2. |
Uniclaro is sentenced to pay Adjupharm EUR 53,990.00 plus interest at 5 percentage points above the German basis rate since 17.01.2023.
|
| 3. |
Uniclaro shall bear the procedural costs.
|
The judgment is not yet final (rechtskräftig). The period for the appeal by each party is one month.
At this stage, the Company management cannot assess the chances of the potential outcome of this these proceedings.
32
Management’s Discussion and Analysis
PROCEEDINGS UNDER CCAA
See "EXECUTIVE SUMMARY - OVERVIEW – CURRENT OPERATIONS IN ISRAEL AND GERMANY" for a summary of the CCAA Proceedings.
Court materials filed in connection with Trichome's CCAA Proceedings can be found at https://www.ksvadvisory.com/insolvency-cases/case/trichome.
THE REGIONAL LABOR COURT - TEL AVIV (BAT YAM) 17419-04-23
On May 10, 2023, IMC Holdings received a notice that a former employee has recently filed a claim with The Regional Labor Court - Tel Aviv against 3 companies, including IMC Holdings. The nature
and details of the claim are still in the preliminary stages, and IMC Holdings is actively working to comprehend the full scope of the allegations.
On April 4, 2024 IMC Holdings filed its Statement of defense.
A preliminary hearing is set for May 6, 2024, before the esteemed Honorable Judge Karin Liber-Levin at the The Regional Labor Court - Tel Aviv (Bat Yam).
At this stage, the Company management cannot accurately assess the potential outcome of the claims or the likelihood of the claims progressing further.
ONTARIO SUPERIOR COURT OF JUSTICE IN CANADA – STATEMENT OF CLAIM
On November 17, 2023, the Company received a copy of a Statement of Claim that was filed in the Ontario Superior Court of Justice in Canada by 35 Oak Holdings Ltd., MW Investments Ltd., 35 Oak
Street Developments Ltd., Michael Wiener, Kevin Weiner, William Weiner, Lily Ann Goldstein-Weiner, in their capacity as trustees of the Weiner Family Foundation (collectively the "MYM Shareholder Plaintiffs")
against the Company and its board of directors, Board and officers, (collectively, the "MYM Defendants").
MYM Shareholder Plaintiffs claims that the MYM Defendants made misrepresentations in its disclosures prior to the Company's transaction with MYM in 2021. The MYM Shareholder Plaintiffs are claiming
damages that amount to approximately $15,000 and aggravated, exemplary and punitive damages in the amount of $1,000.
The Company has reviewed the complaint and believes that the allegations are without merit.
The Company, together with some of the Defendants brought, on February 22, 2024, a preliminary motion to strike out several significant parts of the claim (the "Motion")
The Motion has not been scheduled by the court.
At this time, the Company's management is of the view that the Motion has merit and is likely to succeed in at least narrowing the scope of the claim against the Company, and that it may also
result in certain of the claims against individuals being dismissed altogether, and if not dismissed narrowed in scope and complexity.
The Company plans to vigorously defend itself against the allegations. At this stage, the Company management cannot assess the chances of the claim advancing or the potential outcome of this these
proceedings.
33
Management’s Discussion and Analysis
OFF-BALANCE SHEET ARRANGEMENTS
IM Cannabis had no off-balance sheet arrangements as of March 31, 2024.
TRANSACTIONS WITH RELATED PARTIES
Transactions with related parties mainly includes enterprises owned by directors or major shareholders and enterprises that have a member of key management in common with us. All of the
transactions have been reviewed and approved by the Board or another independent committee of the board.
| • |
On April 2, 2019, IMC Holdings and Focus entered into an option agreement (the “Focus Agreement”) pursuant to which IMC Holdings acquired an option to purchase, at its sole discretion and in
compliance with Israeli cannabis regulation, all of the ordinary shares held by Messrs. Shuster and Gabay held in Focus at a price equal to NIS 765.67 per ordinary share until April 2029. On November 30, 2023, IMC Holdings sent a request
letter to approve IMC Holding’s exercise of the option and on February 25, 2024, IMCA's approval was obtained. Effective February 27, 2024, IMC Holdings acquired 74% of the ordinary shares of Focus.
|
| • |
The Company is a party to Indemnification Agreement with certain directors and officers of the Company and Trichome to cover certain tax liabilities, interest and penalties arising from the Trichome Transaction. See “Risk Factors - Tax Remittance” section of the MD&A.
|
| • |
On October 12, 2023, Oren Shuster, the CEO loaned an amount of NIS 500 thousand (approximately $170) to IMC Holdings. The participation of the CEO constituted a “related party transaction”, as such term is defined in MI 61-101 and would
require the Company to receive minority shareholder approval for and obtain a formal valuation for the subject matter of, the transaction in accordance with MI 61-101, prior to the completion of such transaction. However, in completing the
loan, the Company has relied on exemptions from the formal valuation and minority shareholder approval requirements of MI 61-101, in each case on the basis that the fair market value of the CEO’s loan did not exceed 25% of the market
capitalization of the Company, as determined in accordance with MI 61-101.
|
Other than the aforesaid transactions noted above, the Company had no other transactions with related parties outside of the Group except those pertaining to transactions with key management
personnel and shareholders in the ordinary course of their employment or directorship.
PROPOSED TRANSACTIONS
There are no proposed transactions as at the date of this MD&A that have not been disclosed.
34
Management’s Discussion and Analysis
CRITICAL ACCOUNTING POLICIES
The consolidated financial statements of the Group have been prepared in accordance with International Financial Reporting Standards ("IFRS"), as issued by the International Accounting Standards
Board ("IASB"). The Group's financial statements have been prepared on a cost basis, except for:
- Financial instruments which are presented at fair value through profit or loss.
- Biological assets which are presented at fair value less cost to sell up to the point of harvest.
In the process of applying the significant accounting policies, the Group has made the following judgments which have the most significant effect on the amounts recognized in the financial
statements:
Functional currency, presentation currency and foreign currency
The functional currency of the Company is the Canadian dollar ("CAD"). The Group determines the functional currency of each Group entity.
Assets, including fair value adjustments upon acquisition, and liabilities of an investee which is a foreign operation, and of each Group entity for which the functional currency is not the
presentation currency are translated at the closing rate at each reporting date. Profit or loss items are translated at average exchange rates for all periods presented. The resulting translation differences are recognized in other comprehensive
income (loss). Upon the full or partial disposal of a foreign operation resulting in loss of control in the foreign operation, the cumulative gain (loss) from the foreign operation which had been recognized in other comprehensive income is
transferred to profit or loss. Upon the partial disposal of a foreign operation which results in the retention of control in the subsidiary, the relative portion of the amount recognized in other comprehensive income is reattributed to
non-controlling interests.
Transactions denominated in foreign currency are recorded upon initial recognition at the exchange rate at the date of the transaction. After initial recognition, monetary assets and liabilities
denominated in foreign currency are translated at each reporting date into the functional currency at the exchange rate at that date. Exchange rate differences, other than those capitalized to qualifying assets or accounted for as hedging
transactions in equity, are recognized in profit or loss. Non-monetary assets and liabilities denominated in foreign currency and measured at cost are translated at the exchange rate at the date of the transaction. Non-monetary assets and
liabilities denominated in foreign currency and measured at fair value are translated into the functional currency using the exchange rate prevailing at the date when the fair value was determined.
JUDGMENTS
Determining the fair value of share-based payment transactions
The fair value of share-based payment transactions is determined upon initial recognition by an acceptable option pricing model. The inputs to the model include share price, exercise price and
assumptions regarding expected volatility, expected life of share option and expected dividend yield.
Variable lease payments that depend on an index:
On the commencement date, the Group uses the index rate prevailing on the commencement date to calculate the future lease payments. For leases in which the Group is the lessee, the aggregate
changes in future lease payments resulting from a change in the index are discounted (without a change in the discount rate applicable to the lease liability) and recorded as an adjustment of the lease liability and the right-of-use asset, only
when there is a change in the cash flows resulting from the change in the index (that is, when the adjustment to the lease payments takes effect).
35
Management’s Discussion and Analysis
ESTIMATES AND ASSUMPTIONS
The preparation of the financial statements requires management to make estimates and assumptions that influence the application of the accounting policies and on the reported amounts of assets,
liabilities, revenues, and expenses. Changes in accounting estimates are reported in the period of the change in estimate.
The key assumptions made in the financial statements concerning uncertainties at the reporting date and the critical estimates computed by the Group that may result in a material adjustment to the
carrying amounts of assets and liabilities within the next financial year are discussed below.
ASSESSMENT OF GOING CONCERN
The use of the going concern basis of preparation of the financial statements. At each reporting period, management assesses the basis of preparation of the financial statements. The going concern
basis of presentation assumes that the Company will continue its operations for the foreseeable future and be able to realize its assets and discharge its liabilities and commitments in the normal course of business.
The Group’s current operating budget includes various assumptions concerning the level and timing of cash receipts from sales and cash outlays for operating expenses and capital expenditures.
Restructuring Plans and actions taken in 2022 and in 2023. The Company’s board of directors approved a cost saving plan, to allow the Company to continue its operations and meet its cash obligations. The cost saving plan consists of cost reduction
due to efficiencies and synergies, which include mainly the following steps: discontinued operations of loss-making activities, reduction in payroll and headcount, reduction in compensation paid to key management personnel (including layoffs of key
executives), operational efficiencies and reduced capital expenditures. In 2024, the company will work for fund and/or debt raising and will continue with cost savings effort as well as increased efficiency.
These conditions raise substantial doubt about the Company’s ability to continue as a going concern. The consolidated financial statements do not include any adjustments relating to the
recoverability and classification of assets or liabilities that might be necessary should the Company be unable to continue as a going concern.
BIOLOGICAL ASSETS
The Group’s biological assets consist of cannabis plants. The Group capitalizes the direct and indirect costs incurred related to the biological transformation of the biological assets between the
point of initial recognition and the point of harvest. The direct and indirect costs of biological assets are determined using an approach similar to the capitalization criteria outlined in IAS 2, Inventory. These costs include the direct cost of
planting and growing materials as well as other indirect costs such as utilities and supplies used in the cultivation process. Indirect labor for individuals involved in the cultivation and quality control process is also included, as well as
depreciation on growing equipment and overhead costs such as rent to the extent it is associated with the growing space. All direct and indirect costs of biological assets are capitalized as they are incurred, and they are all subsequently recorded
within the line item cost of revenues on the Group’s statements of profit or loss and other comprehensive income in the period that the related product is sold. The Group then measures the biological assets at fair value less cost to sell up to the
point of harvest, which becomes the basis for the cost of inventory after harvest. The fair value is determined using a model which estimates the expected harvest yield in grams for plants currently being cultivated, and then adjusts that amount
for the expected selling price per gram and also for any additional costs to be incurred (e.g., post-harvest costs). The net unrealized gains or losses arising from changes in fair value less cost to sell during the period are included in the gross
profit for the related period and are recorded in a separate line on the face of the Group’s statements of profit or loss and other comprehensive income. Determination of the fair values of the biological assets requires the Group to make
assumptions about how market participants assign fair values to these assets. These assumptions primarily relate to the level of effort required to bring the cannabis up to the point of harvest, costs to convert the harvested cannabis to finished
goods, sales price, risk of loss, expected future yields from the cannabis plants and estimating values during the growth cycle. The Group accretes fair value on a straight-line basis according to stage of growth (e.g., a cannabis plant that is 50%
through its growing cycle would be ascribed approximately 50% of its harvest date expected fair value, subject to wastage adjustments).
36
Management’s Discussion and Analysis
The fair value of biological assets is categorized within Level 3 of the fair value hierarchy. For the inputs and assumptions used in determining the fair value of biological assets. The Group’s
estimates are, by their nature, subject to change and differences from the anticipated yield will be reflected in the gain or loss on biological assets in future periods.
As of March 31, 2024 and 2023, the Company does not hold biological assets.
BUSINESS COMBINATIONS AND GOODWILL
Business combinations are accounted for by applying the acquisition method. The cost of the acquisition is measured at the fair value of the consideration transferred on the acquisition date with
the addition of non-controlling interests in the acquiree. In each business combination, the Company chooses whether to measure the non-controlling interests in the acquiree based on their fair value on the acquisition date or at their
proportionate share in the fair value of the acquiree's net identifiable assets. Direct acquisition costs are carried to the statement of profit or loss as incurred.
In a business combination achieved in stages, equity interests in the acquiree that had been held by the acquirer prior to obtaining control are measured at the acquisition date fair value while
recognizing a gain or loss resulting from the revaluation of the prior investment on the date of achieving control. Contingent consideration is recognized at fair value on the acquisition date and classified as a financial asset or liability in
accordance with IFRS 9. Subsequent changes in the fair value of the contingent consideration are recognized in profit or loss. If the contingent consideration is classified as an equity instrument, it is measured at fair value on the acquisition
date without subsequent remeasurement.
Goodwill is initially measured at cost which represents the excess of the acquisition consideration and the amount of non-controlling interests over the net identifiable assets acquired and
liabilities assumed. If the resulting amount is negative, the acquirer recognizes the resulting gain on the acquisition date.
IMPAIRMENT OF FINANCIAL ASSETS
The Group evaluates at the end of each reporting period the loss allowance for financial debt instruments measured at amortized cost. The Group has short-term financial assets, principally trade
receivables, in respect of which the Group applies a simplified approach and measures the loss allowance in an amount equal to the lifetime expected credit losses. The impairment loss, if any, is recognized in profit or loss with a corresponding
allowance that is offset from the carrying amount of the assets.
37
Management’s Discussion and Analysis
IMPAIRMENT OF NON-FINANCIAL ASSETS
The Group evaluates the need to record an impairment of non-financial assets whenever events or changes in circumstances indicate that the carrying amount is not recoverable. If the carrying amount
of non-financial assets exceeds their recoverable amount, the assets are reduced to their recoverable amount. The recoverable amount is the higher of fair value less costs of sale and value in use. In measuring value in use, the expected future
cash flows are discounted using a pre-tax discount rate that reflects the risks specific to the asset. The recoverable amount of an asset that does not generate independent cash flows is determined for the cash-generating unit to which the asset
belongs. Impairment losses are recognized in profit or loss. An impairment loss of an asset, other than goodwill, is reversed only if there have been changes in the estimates used to determine the asset's recoverable amount since the last
impairment loss was recognized. Reversal of an impairment loss, as above, shall not be increased above the lower of the carrying amount that would have been determined (net of depreciation or amortization) had no impairment loss been recognized for
the asset in prior years and its recoverable amount. The reversal of impairment loss of an asset presented at cost is recognized in profit or loss.
The following criteria are applied in assessing impairment of these specific assets:
The Group reviews goodwill for impairment once a year, on December 31, or more frequently if events or changes in circumstances indicate that there is an impairment.
Goodwill is tested for impairment by assessing the recoverable amount of the cash-generating unit (or group of cash-generating units) to which the goodwill has been allocated. The Company
identified the operations in Israel, Canada, and Europe as three separate cash-generating units.
An impairment loss is recognized if the recoverable amount of the cash-generating unit (or group of cash-generating units) to which goodwill has been allocated is less than the carrying amount of
the cash-generating unit (or group of cash-generating units). Any impairment loss is allocated first to goodwill. Impairment losses recognized for goodwill cannot be reversed in subsequent periods.
LEGAL CLAIMS
A provision for claims is recognized when the Group has a present legal or constructive obligation as a result of a past event, it is more likely than not that an outflow of resources embodying
economic benefits will be required by the Group to settle the obligation and a reliable estimate can be made of the amount of the obligation.
PUT OPTION GRANTED TO NON-CONTROLLING INTERESTS
When the Group grants non-controlling interests a put option, the non-controlling interests are classified as a financial liability and are not accorded their share in the subsidiary's earnings. At
each reporting date, the financial liability is measured on the basis of the estimated present value of the consideration to be transferred upon the exercise of the put option / based on the fair value of the consideration. Changes in the amount of
the liability are recorded in profit or loss.
38
Management’s Discussion and Analysis
DEFERRED TAX
Deferred taxes are computed in respect of temporary differences between the carrying amounts in the financial statements and the amounts attributed for tax purposes. Deferred taxes are measured at
the tax rate that is expected to apply when the asset is realized or the liability is settled, based on tax laws that have been enacted or substantively enacted by the reporting date.
Deferred tax assets are reviewed at each reporting date and reduced to the extent that it is not probable that they will be utilized. Deductible carry forward losses and temporary differences for
which deferred tax assets had not been recognized are reviewed at each reporting date and a respective deferred tax asset is recognized to the extent that their utilization is probable.
Deferred taxes in respect of investment property that is held with the objective of recovering substantially all of the economic benefits embedded in the investment property through sale and not
through use are measured in accordance with the expected manner of recovery of the base asset, on the basis of sale rather than use.
When the Company owns an investment in a single property company and the manner in which the Company expects to dispose of the investment is by selling the shares of the property company rather
than by selling the property itself, the Company recognizes deferred taxes for both inside temporary differences arising from the difference between the carrying amount of the property and its tax basis, and for outside temporary differences
arising from the difference between the tax basis of the investment and the Company's carrying amount of the net assets of the investment in the consolidated financial statements. Taxes that would apply in the event of the disposal of investments
in investees have not been considered in computing deferred taxes, if the disposal of the investments in investees is not probable in the foreseeable future. Also, deferred taxes that would apply in the event of distribution of earnings by
investees as dividends have not been considered in computing deferred taxes, since the distribution of dividends does not involve an additional tax liability or since it is the Company's policy not to initiate distribution of dividends from a
subsidiary that would trigger an additional tax liability. Taxes on income that relate to distributions of an equity instrument and to transaction costs of an equity transaction are accounted for pursuant to IAS 12. Deferred taxes are offset if
there is a legally enforceable right to offset a current tax asset against a current tax liability and the deferred taxes relate to the same taxpayer and the same taxation authority.
SHARE-BASED PAYMENTS
The Company uses the Black-Scholes option pricing model in determining the fair value of Options issued to employees. In estimating fair value, the Company is required to make certain assumptions
and estimates such as the expected life of the options, volatility of the Company’s future share price, the risk-free rate, future dividend yields and estimated forfeiture rates at the initial grant date.
ESTIMATED USEFUL LIVES AND DEPRECIATION/AMORTIZATION OF PROPERTY AND EQUIPMENT, AS WELL AS INTANGIBLE ASSETS
Property, plant and equipment are measured at cost, including directly attributable costs, less accumulated depreciation, accumulated impairment losses and excluding day-to-day servicing expenses.
Cost includes spare parts and auxiliary equipment that are used in connection with plant and equipment. A part of an item of property, plant and equipment with a cost that is significant in relation to the total cost of the item is depreciated
separately using the component method.
Depreciation of property, plant and equipment is dependent upon estimates of useful lives and residual values which are determined through the exercise of judgement and calculated on a
straight-line basis over the useful lives of the assets at annual rates.
39
Management’s Discussion and Analysis
LEASES
Judgment is used in determining the value of the Company’s right-of-use assets and lease liabilities. The value determined for the Company’s right-of-use assets and lease liabilities can be
materially different based on the discount rate selected to present value the future lease payments as well as the likelihood of the Company exercising extensions, termination, and/or purchase options. The discount rate used to present value the
future lease payments over the life of the lease is based on the Company’s incremental borrowing rate at inception of the lease. This rate is determined by the Company using judgment.
In determining the value of the Company’s right-of-use assets and lease liabilities, the Company assesses future business plans to determine whether to include certain extension options noted in
the lease agreement.
If there is no interest rate implicit in the lease agreement, the Company uses a discount rate that would be charged to a similar borrower, with similar risk characteristics, in a mortgage loan to
purchase the leased facility. This discount rate is used to present value the future lease payments in determining the right-of-use asset and lease liability values at inception of the leases.
DETERMINING THE FAIR VALUE OF AN UNQUOTED FINANCIAL ASSETS AND LIABILITIES
The fair value of unquoted financial assets in Level 3 of the fair value hierarchy is determined using valuation techniques, generally using future cash flows discounted at current rates applicable
for items with similar terms and risk characteristics. changes in estimated future cash flows and estimated discount rates, after consideration of risks such as liquidity risk, credit risk and volatility, are liable to affect the fair value of
these assets.
REVENUE RECOGNITION
Revenue from contracts with customers is recognized when the control over the goods or services is transferred to the customer. The transaction price is the amount of the consideration that is
expected to be received based on the contract terms, excluding amounts collected on behalf of third parties (such as taxes). In determining the amount of revenue from contracts with customers, the Group evaluates whether it is a principal or an
agent in the arrangement. The Group is a principal when the Group controls the promised goods or services before transferring them to the customer. In these circumstances, the Group recognizes revenue for the gross amount of the consideration. When
the Group is an agent, it recognizes revenue for the net amount of the consideration, after deducting the amount due to the principal.
REVENUE FROM THE SALE OF GOODS
Revenue from the sale of cannabis products is generally recognized at a point in time when control over the goods have been transferred to the customer. Payment is typically due prior to or upon
delivery and revenue is recognized upon the satisfaction of the performance obligation. The Group satisfies its performance obligation and transfers control upon delivery and acceptance by the customer.
40
Management’s Discussion and Analysis
Variable consideration:
The Group determines the transaction price separately for each contract with a customer. When exercising this judgment, the Group evaluates the effect of each variable amount in the contract,
taking into consideration discounts, penalties, variations, claims, and non-cash consideration. In determining the effect of the variable consideration, the Group normally uses the "most likely amount" method described in the Standard. Pursuant to
this method, the amount of the consideration is determined as the single most likely amount in the range of possible consideration amounts in the contract. According to the Standard, variable consideration is included in the transaction price only
to the extent that it is highly probable that a significant reversal in the amount of revenue recognized will not occur when the uncertainty associated with the variable consideration is subsequently resolved.
Bill-and-hold arrangements:
Due to strict regulations of security, storage and handling large quantities of cannabis products, the Group's customers may request the Group to retain physical possession of a sold product until
it is delivered to the customer at a future point in time. Revenue from bill-and-hold sales is recognized before the product is physically delivered to the customer when all of the following criteria are met:
| a. |
The reason for the bill-and-hold arrangement is substantive (for example, the customer has requested the arrangement);
|
| b. |
The product is identified separately as belonging to the customer;
|
| c. |
The product currently is ready for physical delivery to the customer;
|
| d. |
The Group does not have the ability to use the product by selling it or delivering it to another customer.
|
CHANGES IN ACCOUNTING POLICIES INCLUDING INITIAL ADOPTION
| a. |
Amendments to IAS 1,"Non Current liabilities with Covenants and Classification of Liabilities as current or non-current":
|
In January 2020 and October 2022, the IASB issued amendments to IAS 1, regarding the criteria for classifying liabilities with covenants as current or non-current. In October 2022 the IASB issued an additional amendment accordingly a Company has to disclose of the book value of the obligation and information on the financial benchmarks as well as facts and circumstances at the end of the reporting period that may lead to the conclusion that the entity will have difficulty meeting the financial covenants.
The Amendment is applicable for annual periods beginning on or after January 1, 2024
| b. |
Amendment to IFRS 16, "LEASES ":
|
In September 2022, the IASB issued an amendment to IFRS 16, " Lease Liability in a Sale and Leaseback" ("IFRS 16"), to provide accounting treatment in the financial statements of the seller-lessee in sale and leaseback transactions when the lease payments are variable lease payments that do not depend on the index or the exchange rate. As part of the amendment, the seller-lessee is required to adopt one of two approaches to measuring the liability for the lease at the time of first recognition of such transactions. The chosen approach constitutes an accounting policy that must be applied consistently.
The Amendment is applicable for annual periods beginning on or after January 1, 2024
41
Management’s Discussion and Analysis
| c. |
Amendments to IAS 7 and IFRS 7, " Supplier Finance Arrangements":
|
In May 2023, the IASB published amendments to International Accounting Standard 7, Statement of Cash Flows, and IFRS 7, Financial Instruments: Disclosures (hereinafter: "the
amendments"), to clarify the characteristics of supplier financing arrangements and to require additional disclosure for these arrangements.
The disclosure requirements in the amendments are intended to assist and enable users of the financial statements to assess the effects of supplier financing arrangements on the
entity's obligations as well as on the entity's cash flows and exposure to liquidity risk.
The Amendment is applicable for annual periods beginning on or after January 1, 2024
FINANCIAL INSTRUMENTS
Financial instruments are measured either at fair value or at amortized cost. The table below lists the valuation methods used to determine fair value of each financial instrument.
|
Financial Instruments Measured at Fair Value
|
Fair Value Method
|
|
|
Derivative assets1
|
Black & Scholes model (Level 3 category)
|
|
|
Warrants liability1
|
Black & Scholes model (Level 3 category)
|
|
|
Investment in affiliates
|
Market comparable (Level 3 category)
|
|
|
Financial Instruments Measured at
Amortized Cost |
||
|
Cash and cash equivalents, trade receivables and other account receivables
|
Carrying amount (approximates fair value due to short-term nature)
|
|
|
Loans receivable
|
Amortized cost (effective interest method)
|
|
|
Trade payables, other accounts payable and accrued expenses
|
Carrying amount (approximates fair value due to short-term nature)
|
Note:
| 1. |
Finance expense (income) include fair value adjustment of warrants, investments, and derivative assets measured at fair value, for the three months ended March 31, 2024 and 2023, amounted to $99 and $3,637,
respectively.
|
42
Management’s Discussion and Analysis
The warrants fair value for March 31, 2024 was measured using the Black & Scholes model with the following key assumptions:
|
Issue date
|
||||||||||||
|
May 2023
|
February 2023
|
May 2021
|
||||||||||
|
Expected volatility
|
48.43
|
%
|
48.43
|
%
|
48.43
|
%
|
||||||
|
Share price (Canadian Dollar)
|
0.72
|
0.72
|
0.72
|
|||||||||
|
Expected life (in years)
|
2.096
|
1.849
|
2.096
|
|||||||||
|
Risk-free interest rate
|
4.12
|
%
|
4.12
|
%
|
4.12
|
%
|
||||||
|
Expected dividend yield
|
0
|
%
|
0
|
%
|
0
|
%
|
||||||
|
Fair value:
|
||||||||||||
|
Per Warrant (Canadian Dollar)
|
$
|
0.031
|
$
|
0.023
|
$
|
0
|
||||||
|
Total Warrants (Canadian Dollar in thousands)
|
$
|
15
|
$
|
122
|
$
|
0
|
||||||
The Group’s exposure to risk for its use of financial instruments are discussed in the Risk Factors.
PROCEDURES AND INTERNAL CONTROL OVER FINANCIAL REPORTING
In accordance with National Instrument 52-109 – Certification of Disclosure in Issuers’ Annual and Interim Filings (“NI
52-109”) and Rule 13a-15 under the United States Securities Exchange Act of 1934, as amended (the “Exchange Act”), the establishment and maintenance of the Company’s disclosure controls and
procedures (“DC&P”) and internal control over financial reporting (“ICFR”) is the responsibility of management.
Internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for
external purposes in accordance with applicable IFRS. Internal control over financial reporting should include those policies and procedures that establish the following:
| ● |
maintenance of records in reasonable detail, that accurately and fairly reflect the transactions and dispositions of assets;
|
| ● |
reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with applicable IFRS;
|
| ● |
receipts and expenditures are only being made in accordance with authorizations of management or the Board; and
|
| ● |
reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of the Company’s assets that could have a material effect on the financial instruments.
|
NI 52-109 requires the CEO and CFO to certify that they are responsible for establishing and maintaining DC&P and ICFR for the Company and have concluded that as at December 31, 2023, those
internal controls have been designed and are effective in providing reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements in accordance with IFRS.
The Company maintains a set of DC&P designed to provide reasonable assurance that information required to be publicly disclosed is recorded, processed, summarized and reported on a timely
basis. As required by NI 52-109 and Exchange Act Rule 13a-15(b), an evaluation of the design and operation of our DC&P was completed as of December 31, 2023 under the supervision and with the participation of management, including our CEO and
CFO using the criteria set forth in the Internal Control - Integrated Framework (2013), issued by the Committee of Sponsoring Organizations of the Treadway Commission. Based upon this evaluation, our CEO and CFO concluded that as at December 31,
2023, the Company’s DC&P and ICFR were effective.
There have been no changes to the Company’s ICFR during the three months ended March 31, 2024 that have materially affected, or are likely to materially affect, the Company’s ICFR.
43
Management’s Discussion and Analysis
LIMITATIONS OF DISCLOSURE CONTROLS AND PROCEDURES AND INTERNAL CONTROL OVER FINANCIAL REPORTING
The Company’s management, including the CEO and CFO, believe that due to inherent limitations, any DC&P or ICFR, no matter how well designed and operated, can provide only reasonable, not
absolute, assurance of achieving the desired control objectives. Further, the design of a control system must reflect the fact that there are resource constraints, and the benefits of controls must be considered relative to their costs. Also,
projections of any evaluation of effectiveness to future periods are subject to the risk that any design will not succeed in achieving its stated goals under all potential future conditions. Accordingly, because of the inherent limitations in a
cost- effective control system, misstatements due to error or fraud may occur and not be detected. Additionally, management is required to use judgment in evaluating controls and procedures.
LIMITATION ON SCOPE OF DESIGN
In accordance with Section 3.3 of National Instrument 52-109 – Certification of Disclosure in Issuers’ Annual and Interim Filings (“NI 52-109”), the Company
has limited the design of its DC&P and ICFR to exclude the controls, policies and procedures of Oranim Plus (the “Excluded Entity”), acquired by the Company or by one of it subsidiaries within 365 days of
the end of the period ended December 31, 2023.
As of March 31, 2024, the Company has implemented its DC&P AND ICFR in all of its subsidiaries.
RESTRUCTURING
Current Israeli law requires prior approval by the IMCA, a unit of the MOH, of the identity of any shareholder owning 5% or more of an Israeli company licensed by the IMCA to engage in
cannabis-related activities in Israel. For a number of reasons, including the opportunity to leverage a network of multiple Israeli licensed producers cultivating under the IMC brand, and in contemplation of a “go-public transaction” to
geographically diversify the Company’s share ownership, IMC Holdings restructured its organization on April 2, 2019 (the “IMC Restructuring”) resulting in the divestiture to Oren Shuster and Rafael Gabay of
its interest in Focus, which is licensed by the IMCA to engage in cannabis-related activity in Israel.
IMC Holdings retains an option with Messrs. Shuster and Gabay to re-acquire the sold interest in Focus at its sole discretion and in accordance with Israeli cannabis regulations, within 10 years of
the date of the IMC Restructuring (the “Focus Agreement”). The Focus Agreement sets an aggregate exercise price equal to NIS 765.67 per share of Focus for a total consideration of NIS 2,756,500, that being
equal to the price paid by Messrs. Shuster and Gabay for the acquired interests in Focus Medical at the time of the IMC Restructuring. On November 30, 2023, IMC Holdings exercised its option to purchase the 74% interest in Focus, held by Oren
Shuster and Rafael Gabay by submitting a request to the IMCA, which approved the transaction on February 25, 2024. IMC Holdings will provide all necessary information and filing to the tax authorities according to the applicable law.
44
Management’s Discussion and Analysis
As part of the IMC Restructuring, on April 2, 2019, IMC Holdings and Focus entered into an agreement, as amended on January 1, 2021 (the “IP Agreement”),
which provides for Focus’s obligation to use the IMC brand for the sale of any cannabis plant and/or cannabis product produced by Focus, either alone or together with other sub-contractors engaged by Focus through the IP Agreement.
Focus is also obligated through a services agreement, as amended on January 1, 2021, (the “Services Agreement”) to use IMC Holdings for certain management
and consulting services including: (a) business development services; (b) marketing services; (c) strategic advisory services; (d) locating potential collaborations on a worldwide basis; and (e) financial analysis services through the Services
Agreement.
Under the IP Agreement, the parties apply an arm’s length royalty as a percentage of the licensees’ net revenues, on a quarterly basis in accordance with a transfer pricing analysis to be updated
from time to time, as consideration for Focus’ use of IMC Holdings’ intellectual property.
Under the Services Agreement, the Parties apply an arm’s length markup on total costs, on a quarterly basis, in accordance with a transfer pricing analysis to be updated from time to time, as
consideration for the provision of such services.
ISRAELI MARKET DEVELOPMENT 2013-2024
According to Israeli Ministry of Health, as of March 2024, there are 135,071 medical cannabis licensed patients in Israel. A monthly prescription of 5,253,000 grams of medical cannabis were
recorded in March 2024 an increase of 478,000 grams of cannabis from March 2023.3
The chart below reflects the growth in licensed medical cannabis patients in Israel between May 2014 to March 2024.4
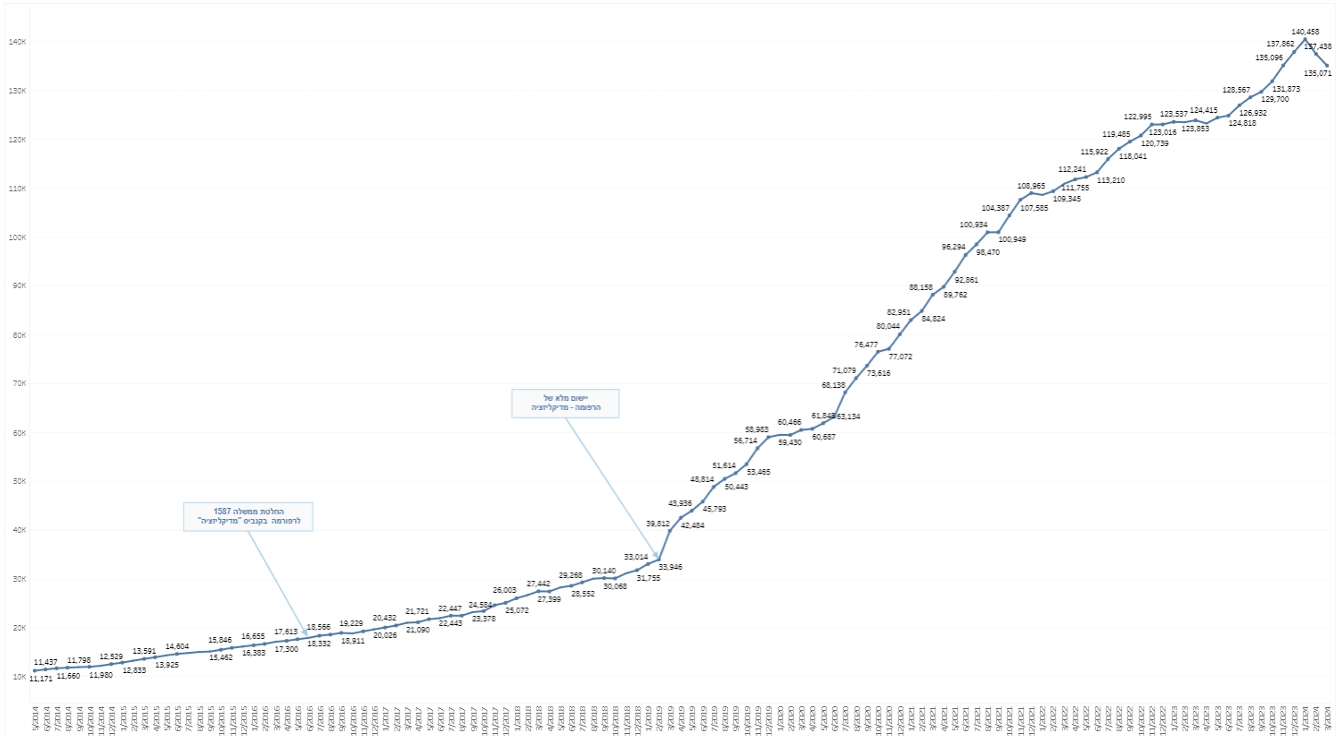
3 Israel Ministry of Health – licensed patients’ data as of April 7 2024 - https://www.gov.il/BlobFolder/reports/licenses-status-march-2024/he/subjects_cannabis_docs_licenses-status-march-2024.pdf
4 Ministry of Health – licensed patients’ data as of April 2024 - www.gov.il/BlobFolder/reports/licenses-status-march-2024/he/subjects_cannabis_docs_licenses-status-march-2024.pdf
45
Management’s Discussion and Analysis
REGULATORY FRAMEWORK IN ISRAEL
In Israel, cannabis is currently defined as a “dangerous drug” according to the Dangerous Drugs Ordinance5 (“DDO”) and the 1961 Single Convention on Narcotic Drugs (“Narcotics Convention”), to which Israel is a signatory. However, both the DDO
and the Narcotics Convention allow for the use of cannabis for medical or research purposes under a supervised and controlled regime. The competent regulatory authority in Israel in all matters concerning the oversight, control and regulation of
cannabis for medical production, consumption, and research in Israel is the IMCA, established by Government Res. No. 3069.6 The
production, distribution and consumption of adult-use recreational cannabis products is currently illegal in Israel.
Patient Medical Consumption
The use of cannabis is allowed for patients and for medical purposes, in respect of certain medical conditions, under a special approval of the MOH. Procedure 1067 of the IMCA sets out a list of medical conditions that are allowed to be treated with medical cannabis products. Such authorized medical conditions are examined
and updated from time to time, and include, among others, cancer, pain, nausea, seizures, muscle spasms, epilepsy, Tourette syndrome, multiple sclerosis, amyotrophic lateral sclerosis, and post-traumatic stress disorder.
Licensing and Authorization for Commercial Activities in the Medical Cannabis Field
In December 2017, the IMCA issued regulations that standardized the licensing process for any cannabis related activity (the “Road Map”).8 Pursuant to the Road Map, each operation in the medical cannabis field, including the propagation, cultivation, products
manufacturing, storage and distribution to licensed pharmacies, and distribution from licensed pharmacies to licensed patients, requires compliance with the provisions of applicable laws, including the procurement of an appropriate license under
the DDO from the IMCA and the maintenance of such license in good standing. Cannabis licenses may not be transferred, exchanged or assigned without the prior approval of the IMCA. The licenses are valid for a period of up to 3 years and can be
renewed with the approval of the IMCA only.
The IMCA has issued a set of directives containing procedures and requirements for applicants for cannabis related activity licenses and has authorized certain entities to issue official
certificates upon compliance with such directives. These directives include (i) Directive 150 (GSP Standard certification); (ii) Directive 151 (GAP Standard certification); (iii) Directive 152 (GMP Standard certification); and (iv) Directive 153
(GDP Standard certification). Regular and periodic examinations are conducted for licensed entities, in order to ensure compliance with the analytical standards and the level of quality required during each of the phases of production and
distribution of medical cannabis.
5 Cannabis is listed in schedule 1 of the Dangerous Drugs Ordinance [New Version], 1973 [ in
English]
https://www.health.gov.il/LegislationLibrary/Samim_01_EN.pdf
6 Israeli Government Res. No. 3609 [in Hebrew], August 7th, 2011
https://www.gov.il/he/departments/policies/2011_des3609
7 Ministry of Health Pharmaceutical Division Policy Number 106 – Licenses for Use of Cannabis
https://www.health.gov.il/hozer/CN_106_2019.pdf (in Hebrew)
8 Directive 107 - Guidelines for the process of licensing the practice of cannabis for medical use,
as amended on October 2020 [Hebrew] - https://www.health.gov.il/hozer/CN_107_2019.pdf
46
Management’s Discussion and Analysis
Medical Cannabis Imports and Exports
The Narcotics Convention governs the import and export of cannabis between member countries. Since Israel is a member country, any export and import of cannabis is subject to the Narcotic
Convention.
In October 2020, the IMCA issued an updated procedure, titled “Guidelines for Approval of Applications for Importation of Dangerous Drug of Cannabis Type for Medical Use and
for Research” (“Procedure 109”), describing the application requirements for cannabis import licenses for medical and research purposes. Therefore, each import of medical cannabis is to be approved by the
IMCA issuing a specific import permit for each imported shipment, rather than a general license for import. An application for import of medical cannabis can be submitted by an entity licensed by the IMCA for the conduct of medical cannabis related
activity. The Israeli government approved the export of pharmaceutical-grade cannabis and cannabis-based products on January 27, 2019,9
and in December 2020, the IMCA published guidelines for the medical cannabis export permit application process.10.
Legalization of Adult-Use Recreational Cannabis and CBD for Non-Medical Purposes in Israel
Currently, adult-use recreational cannabis use in Israel and CBD for non-medical use is illegal. In November 2020, an Israeli government committee responsible for advancing the cannabis market
reform published a report supporting and recommending the legalization of adult-use recreational cannabis in Israel. The Israeli parliament dissolved since then without applying the committee’s’ recommendations and all legislative initiatives were
suspended. However, the new government, formed on June 13, 2021, declared, and settled in the coalition agreement, its commitment to legalization of adult-use recreational cannabis. Since the formation of the new government, several legislative
initiatives were filed, including for the decriminalization of the possession of cannabis for individual recreational adult-use and the legalization of CBD for non-medical use. In February 2022, a Ministry of Health committee contemplated the
legality of CBD and published its recommendation that CBD should be excluded from the DDO. The main recommendations of the committee were adopted by the Minister of Health, however, to date, the Minister has not enacted an order directing that CBD
be removed from the DDO. On April 1, 2022, new regulations came into force which deemed the previously criminal offences of cannabis possession and use for self-consumption into administrative offences, which do not impact a criminal record, and
limited the penalty to a monetary fine only.
Previous Regime and Price Control
Until September 2019, under the previous regime, patients licensed for consumption of medical cannabis products by the IMCA received all of their medical cannabis products
authorized under their respective licenses at a fixed monthly price of NIS 370, regardless of each patient’s authorized amount. Since September 2019, under the new regime, licenses to patients were no longer entitling them for such fixed monthly
price. However, some medical cannabis patient licenses granted under the previous regime remain valid, entitling their holders to receive medical cannabis products pursuant to the price controls and supplier restrictions of the former regime. All
licenses under the previous regime expired in Q1 2022.
9 Directive 4490 [Hebrew] - https://www.gov.il/he/departments/policies/dec4490_2019
10 Directive 110, December 2020 [Hebrew] - https://www.health.gov.il/hozer/CN_110.pdf
47
Management’s Discussion and Analysis
Regulatory Reform from Licenses to Prescriptions for Medical Treatment of Cannabis
In August 2022, the MOH published a draft outline of the transition reform from licenses to prescriptions for medical treatment of cannabis (the “Proposed Outline”). On June 13, 2023, the health committee of the Knesset approved The Dangerous Drugs Regulations (Amendment), 2023 (hereinafter referred to as the "Regulations Amendment""), which entail a model change from issuing licenses to prescriptions permits following the publication of the Proposed Outline11. The Regulations Amendment allows accessibility and significant bureaucratic relief for patients. The purpose of the new prescription model (as defined below) is to enable qualified specialist doctors
(excluding general practitioner, family physician, internal physician and pediatrician) to write prescriptions for medical cannabis for patients under the supervision of health care providers (widely known as Kupat Holim), without requiring a usage
license from the Ministry of Health (hereinafter referred to as "The New Prescription Model").
The main changes in the Regulations Amendment are: (i) any specialized doctor can issue permits without the need for specialized training; (ii) the permits for the use of
cannabis will be in the form of prescriptions, and not in the form of licenses from the MOH as the current framework requires; (iii) cannabis products can be sold in any pharmacy, and not only in pharmacies that have received a special permit from
the IMCA and a license from the MOH. The Regulations Amendment will come into effect within 180 days from the date of their publication. To the best of the Company's knowledge, the indications approved as part of the Regulations Amendment encompass
various conditions, such as oncological diseases, active inflammatory bowel disease, AIDS, Multiple Sclerosis, Parkinson's disease, Tourette syndrome, epilepsy, autism, and dementia.
On December 8, 2023 the Company announced a 3-month delay of the anticipated medical cannabis reform announced by the Israeli ministry of health on August 7, 2023 (the "Reform"). Due to the Israel-Hamas war, the anticipated implementation of the medical cannabis regulatory reform, originally scheduled for December 29, 2023, has been postponed by three months. The new regulations
were designed to alleviate many of the stringent restrictions in the sector, thereby enhancing access to medical cannabis for patients.
On April 1, 2024 the Company announced the implementation of the medical cannabis regulatory reform in Israel as of April 1, 2024.
The Reform will be implemented in phases, as approved, and announced by the Israeli Ministry of Health. The key aspects of the initial phase, commencing today, April , 2024,
are as follows:
1. Change in the prescription process: patients with a wide range of diseases and medical conditions from Oncology to Parkinsons will no longer be required to obtain a license
to receive medical cannabis. Patients will receive a prescription similar to those for other prescription medications. Pain and PTSD are not included in the Reform yet.
2. Medical cannabis will now be prescribed through the HMO's, Israel's public healthcare system: until the Reform, cannabis could not be prescribed through the HMO's which
cover the majority of the Israeli population.
11 [Hebrew] - https://www.gov.il/he/Departments/policies/reform-of-drug-prescription
48
Management’s Discussion and Analysis
3. The number of prescribing physicians is expected to increase: as of today, HMO physicians, who are dully trained and certified within their field of expertise, can prescribe
medical cannabis as a first line treatment, as opposed to a last resort, based on medical discretion for the approved indications.
4. The cost for prescription is anticipated to be reduced: the Ministry of Health limited the cost for a medical cannabis prescription.
For the full report published by the Ministry of Health see (in Hebrew)- https://www.health.gov.il/hozer/mmk152_2016.pdf.
“Anti-Dumping” investigation into cannabis imports from Canada
A notice on the Israeli Government’s website dated January 18, 2024, was addressed to 10 different Canadian cannabis producers: Village Farms International, Organigram Holdings, Tilray Canada, Hexo
Corp (owned by Tilray), The Green Organic Dutchman, Canopy Growth Corporation, SNDL Inc., Cronos Group, Auxly Cannabis Group, Decibel Cannabis, and all the medical cannabis manufacturers in Canada who export their goods to Israel.
The Commissioner for Trade Levies at the Ministry of Economy and Industry, announced by virtue of his authority according to Section 24(d) of the Law on Trade Levies and Defence Measures, 5591 –
1991, of his decision to open an investigation on his own initiative into the export import of cannabis from Canada, after he found that special circumstances of actual damage exist or the probability of actual damage to the local manufacturing
industry and a causal link between the imported imports and said damage. The notice also included a letter sent to Michael Mancini, the Chief Commercial Counselor with the Embassy of Canada, informing them of the investigation, dated January 15,
2024.The Ministry of Economy and Industry issued a formal notice to the public to respond to questionnaires regarding the "Anti-Dumping" investigation.
Further to several requests received from the parties and in accordance with section 27(b) of the Law on Trade Levies and Defense Measures, 1991 which states that "The Commissioner may, for special
reasons that shall be recorded, extend the period specified in subsection (a) by an additional period that shall not exceed 30 days." (the emphasis is not in the original), the Commissioner decided that special conditions exist for extending the
deadline for the submission of the required materials as part of the investigation into the export of medical cannabis to Israel from Canada for 10 days until March 10, 2024, Due to constraints presented by the parties following the "Iron Swords"
war, mainly significant delays in the preparation of the materials due to the absence of many workers as part of the extensive recruitment in Israel for the reserve service at this time and due to the unique complexity of the Israeli cannabis
market where many players are required to submit data both as producers and importer. The Company has submitted the relevant questionnaires regarding its subsidiaries Focus and IMC Pharma, which are included in the investigation and for its
subsidiary Rosen High Way.
49
Management’s Discussion and Analysis
REGULATORY FRAMEWORK IN GERMANY
On March 10, 2017, the German federal government enacted bill Bundestag- Drucksache 18/8965 – Law amending narcotics and other regulations that amended existing narcotics legislation to recognize
cannabis as a form of medicine and allow for the importation and domestic cultivation of medical cannabis products. Under the updated legislation, cannabis is listed in Annex 3 to the Federal Narcotics Act (“BtMG”)
as a “marketable narcotic suitable for prescription”. Legalization in Germany applies only to cannabis for medicinal purposes under state control in accordance with the Narcotic Convention. Currently, the production, distribution, exportation and
importation of medical cannabis products in Germany is legal, subject to regulations and licensing requirements, while operations involving adult-use recreational cannabis products remain illegal. Nevertheless, current German government has
declared in the coalition agreement its intention to open up the German market also in the adult-use recreational market. In October 2022, a key points paper12 on the controlled supply of cannabis to adults for consumption purposes, although a restructuring of the existing regulatory framework on cannabis in general is also discussed, published by the cabinet, which is
to be submitted to the European Union Commission for a preliminary legal examination. In this respect, the Federal Government intends to issue a declaration of interpretation with regard to existing international agreements governing the adult-use
recreational cannabis usage, and to submit a draft law to the European Union Commission within the framework of a notification. After a long political debate, the German Bundestag approved the federal government's draft law "on the controlled use
of cannabis" (BT Drs. 20/870413, BT Drs. 20/876314, BT-Drs. 20/1042615) on Friday, 23 February 2024. It can be assumed that the
draft will also be passed without further hurdles in the German Bundesrat at the end of March and will then essentially come into force on 1 April 2024 in accordance with Art. 15 of the draft. Some components of Article 1 of the draft, which deals
with so-called consumer cannabis, will not come into force until later (according to Art. 15 para. 2 of the draft, for example, not until 1 July 2024). This also has direct consequences for medicinal cannabis, which is the subject matter of Art. 2
(Medical Cannabis Act - MedCanG) and 3 (BtMG) of the draft. With the entry into force of the draft law, cannabis is no longer a narcotic by definition and is therefore no longer subject to the BtMG. The definition in Annex 3 of the BtMG will be
replaced by that in Section 2 MedCanG: "Cannabis for medical purposes: plants, flowers and other parts of plants belonging to the genus Cannabis that are grown for medical purposes under state control in
accordance with Articles 23 and 28(1) of the Single Convention on Narcotic Drugs of 1961 of 30 March 1961 (Federal Law Gazette 1973 II p. 1354), as well as delta-9-tetrahydrocannabinol including dronabinol and preparations of all the
aforementioned substances". However, the narcotics regulations will be replaced by comparable regulations and authorisations. The Federal Institute for Drugs and Medical Devices (BfArM) will remain responsible for the latter as a higher
federal authority. From a regulatory perspective, medicinal cannabis will remain a medicinal product or an active pharmaceutical ingredient even after the amendment of the law, meaning that the requirements under medicinal product law will remain
in place. As a result, the marketing of irradiated products will continue to require a marketing authorisation in accordance with the Ordinance on Medicinal Products Treated with Radioactive or Ionising Radiation (AMRadV). Only the narcotics
licence pursuant to Section 3 BtMG will be replaced by a new licence pursuant to the Medicinal Cannabis Act (MedCanG) (see Section 1), which, however, largely corresponds to the previous provisions of the BTMG with regard to the application process
and general regulations. However, there are the following differences that are new due to the entry into force: Medicinal cannabis no longer has to be stored and transported like a narcotic. The corresponding safety precautions no longer apply,
meaning that compliance with the provisions of pharmaceutical law is sufficient. The so-called semi-annual reports will be replaced by annual reports. The requirements for the person responsible for medicinal cannabis are slightly reduced compared
to those for narcotics. In future, a prescription will be possible without a prescription for narcotics. A normal prescription will suffice. This is just an overview - you are welcome to comment on this in more detail separately. In order to
differentiate, reference is made below to the respective upcoming changes, if relevant at the respective point. However, it is likely to be of great importance that the cultivation of medicinal cannabis based on Section 17 MedCanG is no longer
subject to public tenders, but - like the trading licence - is ultimately subject to a two-stage authorisation (at state level with regard to the pharmaceutical regulations and at federal level with regard to the fact that it is medicinal
cannabis).
12 https://www.bundesgesundheitsministerium.de/fileadmin/Dateien/3_Downloads/Gesetze_und_Verordnungen/GuV/C/Kabinettvorlage_Eckpunktepapier_Abgabe_Cannabis.pdf (in
German language).
13 https://dserver.bundestag.de/btd/20/087/2008704.pdf (in German
language).
14 https://dserver.bundestag.de/btd/20/087/2008763.pdf (in German language).
15 https://dserver.bundestag.de/btd/20/104/2010426.pdf (in German
language).
50
Management’s Discussion and Analysis
Medical cannabis in Germany must comply with the corresponding monographs of the German and European pharmacopoeia. Currently, there are only (non-harmonised) national pharmacopoeial monographs for
cannabis flowers (e.g. in the German Pharmacopoeia (Deutsches Arzneibuch (DAB)) and cannabis extracts (DAB) in the EU. The Committee on Herbal Medicinal Products (HMPC) as the European Medicines Agency’s (EMA) committee responsible for compiling
and assessing scientific data on herbal substances, preparations and combinations, announced that in view of uniform EU quality requirements (including with respect to import and export of cannabis), further European Pharmacopoeia (Ph. Eur.)
Cannabis monographs are in preparation.
The European Pharmacopoeia (Ph. Eur.) Suppl. 11.5 is currently available and contains the new Ph. Eur. Monograph on cannabis flowers and the new Ph. Eur. Monograph on Cannabidiol (CBD). According
to the current status, the Ph. Eur. Monograph on Cannabis Flowers will replace the currently existing national monographs (NL, DK, D and CH) from the official implementation date (1 July 2024). However, there are various authorities that only want
to refer to this monograph from the official translation into German in Germany. The new monograph on cannabis flowers includes Starting materials for the production of extracts, medicinal products that can be prescribed as such (herbal medicinal
products) that are taken by patients by inhalation or oral administration. There are not entirely irrelevant changes compared to the German monograph.
All BtMG permit applications must specify the strains and estimated quantities of medical cannabis involved and any subsequent changes must be reported to the Federal Opium Agency of Germany. The
same applies with regard to Sections 7, 8 MedCanG in relation to the future authorisation to trade in medicinal cannabis, although it is now apparent that no expected annual quantities are to be specified. However, it can be assumed that the BfArM
will nevertheless enquire about these due to the (albeit somewhat reduced compared to the BtMG) reporting obligations in Sections 16 and 17 MedCanG and the Foreign Narcotics Trade Regulation, which remains applicable (see Section 14 MedCanG).
Unlike cannabis, CBD is not subject to German narcotics laws, unless it is synthetic CBD that has been included as a substance that can be prescribed and marketed in Annex 3 of the BtMG, which may
or may not be subject to German drug laws depending on its use and dosage. Annex 1 of the Ordinance on the Prescription of Medicinal Products stipulates that CBD is in principle subject to prescription but does not specify a minimum quantity or a
specific dosage form. However, a distinction must be made between consumable products that naturally contain CBD and those that are infused with CBD extract; the European Commission considers the latter to be a type of “food” and has recently
indicated that all current novel food applications have at least insufficient data on safety and therefore none of the applications can currently lead to approval. In light of the above, various products containing CBD can be found in the German
market. There are currently various court decisions that problematize CBD in food (specifically food supplements) and in cosmetics (specifically: mouth oil). On the one hand, CBD is regarded as a medicinal substance and/or as a novel food subject
to authorization and therefore unsuitable for use in a foodstuff, and on the other hand as unsuitable for cosmetic use in the mouth, as CBD would ultimately be consumed in this case (like a foodstuff).
51
Management’s Discussion and Analysis
Cultivation in Germany and Distribution of Medical Cannabis Cultivated in Germany
The Federal Opium Agency of Germany’s Federal Institute for Drugs and Medical Devices (“BfArM”) formed a cannabis division (the “Cannabis Agency”) to oversee cultivation, harvesting, processing, quality control, storage, packaging and distribution to wholesalers, pharmacists and manufacturers. The Cannabis Agency also regulates pricing of German-produced
medical cannabis products and serves as an intermediary of medical cannabis product sales between manufacturers, wholesalers and pharmacies on a non-profit basis so far. In late 2018, the Cannabis Agency issued a call for tenders to award licenses
for local medical cannabis cultivation and distribution of German-cultivated medical cannabis products (the “German Local Tender”). The Cannabis Agency would serve as an intermediary in the supply chain
between such cultivation and distribution. In April 2019, three licenses for local cultivation were granted. In consequence three companies in Germany cultivate on behalf of the Cannabis Agency of the BfArM. Each license permitted the holder to
grow up to 200kg per year for total production of 2,600kg per year collectively from the 13 cultivation lots and 10,400kg over the four-year license period. In July 2021, the BfArM launched the state sale of cannabis grown in Germany. Since then,
pharmacies have been able to purchase medical cannabis in pharmaceutical drug quality for the supply of patients from the BfArM via the portal www.cannabisagentur.de. The sale from the BfArM to pharmacies is at a price of 4.30 euros per gram.
The Cannabis Agency has no influence on the actual retail price of medical cannabis products and is not responsible for the import of medical cannabis products and will therefore neither purchase
nor distribute imported medical cannabis products. As a wholesaler, the Cannabis Agency sells German-based medical cannabis products in its own name.
Future Situation:
With the entry into force of the above-mentioned draft, the granting of licences for domestic cultivation is governed by §§ 4 et seq. MedCanG. The previously time-consuming tendering and awarding
of contracts for the domestic cultivation of cannabis for medical purposes by the Cannabis Agency and the subsequent purchase and distribution of the domestic harvest yields by the Cannabis Agency from the economic operators determined in the
course of the tendering procedure will no longer be necessary in future.
Import volumes and procedures
The current regime permits the importation of cannabis plants and plant parts for medicinal purposes under state control subject to the requirements under the Narcotic Convention, according to
which, Germany must estimate the expected demand of medical cannabis products for medical and research purposes for the following year and report such estimates to the International Narcotics Control Board.
As a prerequisite to obtaining a German import license, the supplier must grow and harvest in compliance with EU-GACP-Guidelines and manufacture in compliance with EU-GMP-Guidelines and
certifications, or alternatively, it is a pure EU-GACP product and the EU-GMP manufacturing steps then take place in Germany. All medical cannabis products imported to Germany must derive from plant material cultivated in a country whose
regulations comply with the Narcotic Convention and must comply with the relevant monographs described in the German and European pharmacopeias. While these requirements also apply to the exportation of medical cannabis products, the current German
regime does not allow domestically cultivated medical cannabis products to be directly sold to commercial entities other than the Cannabis Agency.
Dispensing Exclusively via Pharmacies
Medical cannabis products imported pursuant to an import license under the BtMG (and under the MedCanG as well) and AMG permits are sold exclusively to pharmacies for final dispensing to patients
on a prescription basis as ‘magistral preparations’, a term used in Europe to refer to medical products prepared in a pharmacy in accordance with a medical prescription for an individual patient. Magistral preparations require certain manufacturing
steps in the pharmacy. Such manufacturing steps of the pharmacist typically include the testing and dosing of pre-packaged cannabis inflorescences (typically referred to as “flos”), medical cannabis products for oral administration (dronabinol),
medical cannabis products for inhalation upon evaporation, and medical cannabis-infused teas. In addition to magistral preparations, medical cannabis products are also marketable as pre-packaged, licensed drugs (e.g. Sativex®).
52
Management’s Discussion and Analysis
NO U.S. CANNABIS-RELATED ACTIVITIES
The Group does not engage in any U.S. cannabis-related activities as defined in Canadian Securities Administrators Staff Notice 51-352 (Revised) – Issuers with
U.S. Marijuana-Related Activities.
The Company has implemented risk management governance processes that are led by the Board, with the active participation of management, and updates its assessment of its business risks on an
annual basis. Notwithstanding, it is possible that the Company may not be able to foresee all the risks that it may have to face. The market in which IM Cannabis currently competes is complex, competitive and changing rapidly, and its business is
subject to risks inherent in a high growth, heavily regulated enterprise, and the Company has identified certain risks pertinent to the Group’s business that may have affected or may affect the Group’s business, financial conditions, results of
operations and cash flows, as further described throughout this MD&A and under “Risk Factors” in the Company’s Annual Report on Form 20-F for the fiscal year ended December 31, 2023 available on the Company’s profile on SEDAR+ at www.sedarplus.com
and on EDGAR at www.sec.gov/edgar (the “Annual Report”). For additional risk factors, readers are directed to the Annual Report. Sometimes new risks emerge, and management may not be able to predict all of them or be able to predict how
they may cause actual results to be different from those contained in forward looking statements. Readers of this MD&A should not rely upon forward looking statements as a prediction of future results.
CREDIT RISK
The maximum credit exposure as of March 31, 2024, is the carrying amount of cash and cash equivalents, trade receivables and other current assets. The Group does not have
significant credit risk with respect to outstanding trade receivables. All cash and cash equivalents are placed with major Israeli financial institutions.
Loan receivable credit risk is managed by each loan separately according to the Company’s policy, procedures and control relating to the borrower’s credit risk management. At
the end of each period, the individual loan values are assessed based on a credit risk analysis.
The expected credit loss analysis is generally based on management’s understanding of the borrower’s experience/integrity, financial health, business plans, capacity, products,
customers, contracts, competitive advantages/disadvantages, and other pertinent factors when assessing credit risk. This would also include the assessment of the borrower’s forecasts as well as taking into consideration any security and/or
collateral the Company has on the outstanding balance.
LIQUIDITY RISK
As of March 31, 2024, the Group's financial liabilities with liquidity risk consist of trade payables and other accounts payable which have contractual maturity dates within one year, bank loans
and, checks receivables and lease liabilities. The Group manages its liquidity risk by reviewing its capital requirements on an ongoing basis. Based on the Group's working capital position at March 31, 2024, management considers liquidity risk to
be high.
53
Management’s Discussion and Analysis
CURRENCY RATE RISK
As of March 31, 2024, a portion of the Company's financial assets and liabilities held in Euro, NIS and USD consist of cash and cash equivalents in the amount of EUR 278
thousand (approximately $407), NIS 3,698 thousand (approximately $1,350), USD 15 thousand (approximately $20), respectively. The Company's objective in managing its foreign currency risk is to minimize its net exposure to foreign currency cash
flows by transacting, to the greatest extent possible, with third parties in NIS. The Company does not currently use foreign exchange contracts to hedge its exposure of its foreign currency cash flows as management has determined that this risk is
not significant at this point of time.
SHARE PRICE RISK
The Group's investments in unlisted shares are sensitive to market price risk arising from uncertainties about future value of these investments. The Group manages the price risk through diversification and by
placing limits on individual and total investment in shares. The Company's Board of directors reviews and approves all decisions related to investments in shares. At the reporting date, the Group's exposure to investments in unlisted shares
measured at fair value was $2,078.
TAX REMITTANCE
The Company is subject to the provisions of the ITA12 and to review by CRA13. The Company files its annual tax compliance based on its interpretation of the ITA and CRA’s guidance. There is no
certainty that the returns and tax position of the Company will be accepted by CRA as filed. Any difference between the Company’s tax filings and CRA’s final assessment could impact the Company’s results and financial position.
There can be no assurance that income tax laws or the interpretation thereof in any of the jurisdictions in which the Company operates will not be changed or interpreted or administered in a manner
which adversely affects the Company and its shareholders. In addition, there is no assurance that CRA will agree with the manner in which the Company calculates taxes payable or that any of the other tax agencies will not change their
administrative practices to the detriment of the Company or its shareholders.
By Notice of Assessment for Excise Tax dated October 23, 2023 and covering the period January 1, 2020 to December 31, 2020, the Company was assessed tax on insurance of $198,687.57, arrears
interest of $36,248.62 and a failure to file penalty of $7,947.49 (collectively, the “2020 Assessment”).
By Notice of Assessment for Excise Tax dated October 23, 2023 and covering the period January 1, 2021 to December 31, 2021, the Company was assessed excise tax on insurance of $72,944.92, arrears
interest of $1,533.75 and a failure to file penalty of $499.48 (collectively, the “2021 Assessment”).
If a person files a Notice of Objection (Excise Tax Act), the CRA cannot take collection action on amounts in dispute until 90 days after the Notice of Decision is sent to that person. However,
interest and penalty continue to accrue on any amount owing.
On November 29, 2023, the Company filed Notices of Objection (Excise Tax Act) to the 2020 Assessment and the 2021 Assessment. Therefore, the CRA cannot take collection action on the amounts noted
above until 90 days after Notices of Decision are sent to the Company.
The Company assess the filed Notices of Objection (Excise Tax Act) to be low to medium complexity. The CRA is currently taking roughly 150 to 300 days to issue a Notice of Decision in respect of a
low to medium complexity Notice of Objection (Excise Tax Act).
The Company assess that it is reasonably possible the Notices of Objection (Excise Tax Act) filed by IM Cannabis Corp. will lead to the 2020 Assessment and 2021 Assessment being vacated.
54
Management’s Discussion and Analysis
CONSOLIDATION OF CERTAIN FINANCIAL RESULTS UNDER IFRS 10 AND MAINTENANCE OF COMMON CONTROL
The Company complies with IFRS 10, which applies a single consolidation model using a definition of “control” that requires an investor (as defined in IFRS 10) to consolidate an investee (as
defined in IFRS 10) where: (i) the investor has power over the investee; (ii) the investor has exposure or rights to variable returns from involvement with the investee; and (iii) the investor can use its power over the investee to affect the
amount of the investor’s returns.
Subsequent to the restructuring of IMC Holdings on April 2, 2019, the Company analyzed the terms of the contractual agreements with Focus in accordance with IFRS 10 to conclude whether it should
continue to consolidate the accounts of Focus in its financial statements.
Under IFRS 10, consolidation occurs when an investor can exercise control over an investee. Control is achieved through voting rights or other evidence of power. Where there are no direct holdings,
under IFRS 10, an investor (as defined in IFRS 10) should consider other evidence of power and ability to unilaterally direct an investee’s (as defined in IFRS 10) relevant activities. In view of the contractual agreements and the guidance in IFRS
10, notwithstanding that the Company has no direct or indirect ownership of Focus Medical, it has sufficient rights to unilaterally direct the relevant activities (a concept known as “de facto control”), mainly due to the following:
| (a) |
the Company receiving economic benefits from Focus (and the terms of the contractual agreements between the Company and Focus cannot be changed without the approval of IMC Holdings);
|
| (b) |
IMC Holdings having the option to purchase the divested 74% interest in Focus held by Oren Shuster, the CEO, director and a promoter of the Company, and Rafael Gabay, a former director and a promoter of the Company;
|
| (c) |
Messrs. Shuster and Gabay each being a director of Focus (while Mr. Shuster concurrently being a CEO, director and substantial shareholder of the Company and Mr. Gabay concurrently being a substantial shareholder of the Company); and
|
| (d) |
the Company providing management and support activities to Focus through a services agreement.
|
Accordingly, under IFRS 10, the Company has “de facto control” over Focus, and therefore consolidates the financial results of Focus in the Company’s financial statements.
Any failure of the Company or Messrs. Oren Shuster and Rafael Gabay to maintain “de facto control” over Focus as defined under IFRS 10 could alter the Company’s consolidation model, potentially
resulting in a material adverse effect on the business, results of operations and financial condition of the Company.
On November 30, 2023, IMC Holdings acted to exercise its option to purchase the divested 74% interest in Focus held by Oren Shuster, and Rafael Gabay by submitting a request to the "IMCA," an
agency operated by the Israeli Ministry of Health that will allow the option exercise. On February 25, 2024, IMCA approved the persons who will be acting on behalf on IMC Holding pursuant to the exercise of the option, allowing to complete the
transaction . On February 26, 2024, IMC Holdings has exercised its option and as of that date, IMC holds 74% of Focus. The Company will continue to consolidate the financial results of Focus in the Company’s financial statements.
55
Management’s Discussion and Analysis
POSSIBLE DIRECT INVOLVEMENT IN THE ISRAELI CANNABIS INDUSTRY
According to current Israeli regulatory medical cannabis framework, any engagement in Cannabis Activities requires receiving the applicable license from the “IMCA”, an agency operated by the
Israeli Ministry of Health, which requires, among other things, pre-approvals by the IMCA (the “IMCA Pre-Approval Requirement”) of the directors, officers and shareholders holding 5% or more of the shares of the license applicant (“Material
Holders”), and of all directors, officers and shareholders that become Material Holders following the grant of the applicable license. Therefore, if the Company will be considered by the IMCA as directly engaged in Cannabis Activities the
aforementioned approvals by the IMCA might apply, on future security holdings, as described above.
Furthermore, any failure of the Company or its shareholders to comply with the IMCA Pre-Approval Requirement may impact the Group’s ability to continue operating in compliance with any licenses to
engage in Cannabis Activities or to renew such licenses. Any inability of the Group to maintain licenses for Cannabis Activities in good standing may result in a material adverse effect on the Group’s business, financial condition, results of
operations and prospects.
COMPANY’S ABILITY TO CONTINUE AS A GOING CONCERN
The Group’s current operating budget includes various assumptions concerning the level and timing of cash receipts from sales and cash outlays for operating expenses and capital expenditures, including cost saving
plans. In 2023 The Company’s board of directors approved a cost saving plan, to allow the Company to continue its operations and meet its cash obligations. The cost saving plan consisted cost reduction due to efficiencies and synergies, included
mainly the following steps: discontinued operations of loss-making activities, reduction in payroll and headcount, reduction in compensation paid to key management personnel (including layoffs of key executives), operational efficiencies and
reduced capital expenditures. Those actions will save costs in 2024 and the company will continue its efforts for efficiency operations.
Despite the cost savings plan and restructuring as described above, the projected cash flows for 2024 indicates that it is uncertain that the Group will generate sufficient funds to continue its operations and meet its
obligations as they become due. The Group continues to evaluate additional sources of capital and financing. However, there is no assurance that additional capital and or financing will be available to the Group, and even if available, whether it
will be on terms acceptable to the Group or in amounts required.
These conditions raise substantial doubt about the Company’s ability to continue as a going concern. The consolidated financial statements do not include any adjustments relating to the
recoverability and classification of assets or liabilities that might be necessary should the Company be unable to continue as a going concern.
CONFLICT AND POLITICAL INSTABILITY IN EASTERN EUROPE
The first part of 2023 has seen significantly higher levels of volatility in global markets due to market participants’ reactions to, and uncertainty surrounding, the magnitude and timing of
government and central bank action to be taken in response to heightened inflation, as well as Russia’s invasion of Ukraine. This volatility has resulted in a decline in the level of activity in the financial markets. Continued market volatility or
uncertainty related to actions taken or to be taken by central banks, a decline in the global macroeconomic outlook, including as a result of Russia’s invasion of Ukraine and the threat, or outbreak of more widespread armed conflict in Eastern
Europe would cause financial market activity to continue to decrease, which would negatively affect the Group’s revenues and capital markets activity.
56
Management’s Discussion and Analysis
CONFLICT AND POLITICAL INSTABILITY IN ISRAEL THE ISRAEL- HAMAS WAR
The Group is vulnerable to the political, economic, legal, regulatory, and military conditions affecting Israel and the Middle East. Armed conflicts between Israel and its neighbouring countries
and territories occur periodically in the region and may adversely affect the Group’s business, results of operations and financial condition. In addition, the Group may be adversely affected by other events or factors affecting Israel such as the
interruption or curtailment of trade between Israel and its trading partners, or any restrictions or pressure on the Group’s partners or customers or others to prevent or discourage them from doing business activities with Israel or Israeli
businesses, a significant downturn in the economic or financial condition of Israel, a significant downgrading of Israel’s internal credit rating, labour disputes and political instability, including riots, uprisings and government failures.
Restrictive laws or policies directed towards Israel or Israeli businesses could have a material adverse effect on the Group’s business, results of operations, financial condition and prospects.
Any armed conflicts, terrorist activities or political instability in the region could adversely affect business conditions, could harm the Group’s results of operations, and could make it more
difficult for us to raise capital. Parties with whom the Group does business may decline to travel to Israel during periods of heightened unrest or tension, forcing the Group to make alternative arrangements when necessary in order to meet our
business partners face to face. In addition, the political and security situation in Israel may result in parties with whom we have agreements involving performance in Israel claiming that they are not obligated to perform their commitments under
those agreements pursuant to force majeure provisions in such agreements. Further, in the past, the State of Israel and Israeli companies have been subjected to economic boycotts. Several countries still restrict business with the State of Israel
and with Israeli companies. These restrictive laws and policies may have an adverse impact on our operating results, financial condition or the expansion of our business.
Furthermore, under Israeli law, citizens and permanent residents of Israel are obligated to perform military reserve duty for extended periods of time and are subject to being called to active duty
at any time under emergency circumstances. In response to increased hostilities, there have been periods of significant call-ups of military reservists.
On October 7, 2023, a war between the terror organization Hamas and Israel began. This war has an impact on the Company's business operations. The Company has also suffered a negative impact in Q1
2024 and there will be a potential positive effect in the medium to long term. The Company has experienced damages to its ability to function, affecting various aspects, including employees, supplies, imports, sales, and more.
While there are damages, it is still too early to fully assess the extent of their impact. It is possible that there will be additional call-ups in the future, which may include officers and key
personnel of the Group’s, which could disrupt business operations for a significant period of time.
JUDICIAL AND LEGISLATIVE REFORMS IN ISRAEL
Israel is undergoing political and social instability relating to the judicial and legislative reforms proposed by the current government, creating certain instability and uncertainty. This
instability which has a certain effect on the activity of the financial markets may cause material impact on the Groups’ ability to operate in the Israeli market, which derives, among other, from: exposure to currency exchange rate and interest
rate, reduced sales due to disruptive days and lower probability for capital investments.
57
Management’s Discussion and Analysis
on August 7, 2023, the Israeli ministry of health announced the anticipated medical cannabis regulatory reform (the "Reform"). The new regulations under the
Reform might remove many of the heavy regulations in the sector, making medical cannabis more accessible to patients as well as boosting export, all of which may materially and positively impact the business, financial condition and results of
operations of the Company, its subsidiaries and Focus. Due to the Israel-Hamas war, the anticipated implementation of the Reform, originally scheduled for December 29, 2023, has been postponed by three months.
On April 1, 2024, the Company announced the implementation of the medical cannabis regulatory reform in Israel on April 1st.
The Reform will be implemented in phases, as approved, and announced by the Israeli Ministry of Health. The key aspects of the initial phase, commencing today, April 1st, are as
follows:
1. Change in the prescription process: patients with a wide range of diseases and medical conditions from Oncology to Parkinsons will no longer be required to obtain a license
to receive medical cannabis. Patients will receive a prescription similar to those for other prescription medications. Pain and PTSD are not included in the Reform yet.
2. Medical cannabis will now be prescribed through the HMO's, Israel's public healthcare system: until the Reform, cannabis could not be prescribed through the HMO's which
cover the majority of the Israeli population.
3. The number of prescribing physicians is expected to increase: as of today, HMO physicians, who are dully trained and certified within their field of expertise, can prescribe
medical cannabis as a first line treatment, as opposed to a last resort, based on medical discretion for the approved indications.
4. The cost for prescription is anticipated to be reduced: the Ministry of Health limited the cost for a medical cannabis prescription.
For the full report published by the Ministry of Health see (in Hebrew)- https://www.health.gov.il/hozer/mmk152_2016.pdf. CCAA PROCEEDINGS
On September 14, 2023 a CCAA Termination Order was granted by the Honourable Justice Osborne (upon service on the Service List of an executed certificate and the above CCAA Proceedings under the Companies Creditors’ Arrangement Act and the Stay Period were terminated without any further act or formality. On September 29th,
2023, Trichome Financial Corp. filed (or was deemed to have filed) an assignment (or a bankruptcy order was made against Trichome Financial Corp.), and Goldhar & Associates Ltd., was appointed as trustee of the estate of the bankrupt by the
official receiver (or the Court). The first meeting of creditors of the bankrupt was held on October 17th, 2023.
As a direct or indirect shareholder of the entities that make up the Trichome Group, the Company is subject to the priorities of other stakeholders in the CCAA proceedings and will likely realize
no return in the restructure of the Trichome Group business.
ENVIRONMENTAL RISKS
The Group’s operations are subject to environmental and occupational safety laws and regulations in certain jurisdictions, concerning, among other things, emissions and discharges to water, air and
land, the handling and disposal of hazardous and nonhazardous materials and wastes, and employee health and safety. The Group incurs ongoing costs and obligations related to compliance with environmental and employee health and safety matters. Any
failure to comply or maintain compliance with environmental and occupational safety laws and regulations may result in additional costs for corrective measures, penalties or restrictions on manufacturing operations and could have a material adverse
effect on the business, results of operations and financial condition of the Group.
58
Management’s Discussion and Analysis
RISKS INHERENT IN THE AGRICULTURAL BUSINESS
The Company’s business involves the growing of cannabis products by third party suppliers, which are agricultural products. As such, the business is subject to the risks inherent in the
agricultural business, such as pests, plant diseases and similar agricultural risks. Although, the third-party cultivators the Company partner with carefully monitor the growing conditions with trained personnel and applicable equipment, there can
be no assurance that natural elements will not have a material adverse effect on the production of its products and results of operations. Any decline in production could have a material adverse effect on the Group’s business, operating results or
financial condition.
Certain statements in this MD&A may contain “forward-looking statements” or “forward-looking information,” within the meaning of applicable Canadian and United States securities legislation
(collectively referred to herein as “forward-looking statements”). All statements other than statements of fact may be deemed to be forward-looking statements, including statements with regard to expected financial performance, strategy and
business conditions. The words “believe”, “plan”, “intend”, “estimate”, “expect”, “anticipate”, “continue”, or “potential”, and similar expressions, as well as future or conditional verbs such as “will”, “should”, “would”, and “could” often
identify forward-looking statements. These statements reflect management’s current expectations and plans with respect to future events and are based on information currently available to management including based on reasonable assumptions,
estimates, internal and external analysis and opinions of management considering its experience, perception of trends, current conditions and expected developments as well as other factors that management believes to be relevant as at the date such
statements are made. No assurance can be given that the expectations in any forward-looking statement will prove to be correct and, as such, the forward-looking statements included in this MD&A should not be unduly relied upon. Forward-looking
statements is by its nature prospective and requires IM Cannabis to make certain assumptions and is subject to inherent risks and uncertainties. All forward-looking statements are provided as of the date of this MD&A. The Company does not
undertake to update any such forward-looking statements whether as a result of new information, future events or otherwise, except as required by law.
Forward-looking statements in this MD&A may include, without limitation, forward-looking statements pertaining to:
| ● |
the Company’s business objectives and milestones and the anticipated timing of execution;
|
| ● |
the performance of the Company’s business, strategies and operations;
|
| ● |
the Company’s intentions to expand the business, operations and potential activities of the Company;
|
| ● |
the Company’s plans to expand its sales channels, distribution, delivery and storage capacity, and reach to medical cannabis patients;
|
| ● |
the competitive conditions of the cannabis industry and the growth of medical or adult-use recreational cannabis markets in the jurisdictions in which the Company operates;
|
| ● |
the competitive conditions of the industry, including the Company’s ability to maintain or grow its market share and maintain its competitive advantages;
|
| ● |
statements relating to the Company’s commitment to responsible growth and compliance with the strictest regulatory environments;
|
59
Management’s Discussion and Analysis
| ● |
the Company’s focus on providing premium cannabis products to medical patients in the jurisdictions in which the Company conducts business and any other jurisdiction in which the Company may conduct business in the future;
|
| ● |
the Company’s plans to amplify its commercial and brand power to become a global high-quality cannabis player;
|
| ● |
the Company’s primary goal of sustainably increasing revenue in its core markets;
|
| ● |
the demand and momentum in the Company’s Israeli and Germany operations;
|
| ● |
how the Company intends to position its brands;
|
| ● |
the efficiencies and synergies of the Company as a global organization with domestic expertise in Israel and Germany;
|
| ● |
expectations that providing high-quality, reliable supply to the Company’s customers and patients will lead to recurring sales;
|
| ● |
expectations related to the Company’s introduction of new Stock Keeping Unit (“SKUs”)
|
| ● |
anticipated cost savings from the reorganization of the Company's and the completion thereof upon the timelines disclosed herein;
|
| ● |
geographic diversification and brand recognition and the growth of the Company’s brands in the jurisdictions that the Company operates in or may expand to;
|
| ● |
expectations related to the Company’s ability to address the ongoing needs and preferences of medical cannabis patients;
|
| ● |
the Company’s retail presence, distribution capabilities and data-driven insights;
|
| ● |
the future impact of the Regulations Amendment regarding the transition reform from licenses to prescriptions for medical treatment of cannabis;
|
| ● |
the Company’s continued partnerships with third party suppliers and partners and the benefits thereof;
|
| ● |
the Company’s ability to achieve profitability in 2024;
|
| ● |
the number of patients in Israel licensed by the Israeli Ministry of Health (“MOH”) to consume medical cannabis;
|
| ● |
expectations relating to the number of patients paying out-of-pocket for medical cannabis products in Germany;
|
| ● |
the anticipated decriminalization or legalization of adult-use recreational cannabis in Israel and Germany;
|
| ● |
expectations related to the demand and the ability of the Company to source premium and ultra-premium cannabis products exclusively and competition in this product segment;
|
| ● |
the anticipated impact of inflation and liquidity on the Company’s performance;
|
| ● |
expectations with respect to the Company’s operating budget and the assumptions related thereto;
|
| ● |
expectations relating to the Company as a going concern and its ability to conduct business under the ordinary course of operations;
|
| ● |
expectations related to the collection the payment awarded in the Judgment and the chances of the claim advancing or the potential outcome of the Test Kits Appeal (as defined herein);
|
| ● |
the continued listing of the Company’s Common Shares in the capital of the Company (“Common Shares”) on the Nasdaq Stock Market (“Nasdaq”) and Canadian
Securities Exchange (“CSE”);
|
| ● |
cannabis licensing in the jurisdictions in which the Company operates;
|
| ● |
the renewal and/or extension of the Company’s licenses;
|
| ● |
the Company’s anticipated operating cash requirements and future financing needs;
|
| ● |
the Company’s expectations regarding its revenue, expenses, profit margins and operations;
|
| ● |
the anticipated Gross Margins, EBITDA and Adjusted EBITDA from the Company’s operations;
|
60
Management’s Discussion and Analysis
| ● |
the expected increase in revenue and margins in its Israeli medical cannabis market activities arising from its acquisitions;
|
| ● |
future opportunities for the Company in Israel, particularly in the retail and distribution segments of the cannabis market;
|
| ● |
future expansion and growth opportunities for the Company in Germany and Europe and the timing of such;
|
| ● |
contractual obligations and commitments; and
|
| ● |
the Company completing the Potential Transaction with Kadimastem (each as defined herein).
|
With respect to the forward looking-statements contained in this MD&A, the Company has made assumptions regarding, among other things:
| ● |
the Company has the ability to achieve its business objectives and milestones under the stated timelines;
|
| ● |
the Company will succeed in carrying out its business, strategies and operations;
|
| ● |
the Company will realize upon its intentions to expand the business, operations and potential activities of the Company;
|
| ● |
the Company will expand its sales channels, distribution, delivery and storage capacity, and reach to medical cannabis patients;
|
| ● |
the competitive conditions of the cannabis industry and the growth of medical or adult-use recreational cannabis in the jurisdictions in which the Company operates;
|
| ● |
the competitive conditions of the industry will be favourable to the Company, and the Company has the ability to maintain or grow its market share and maintain its competitive advantages;
|
| ● |
the Company will commit to responsible growth and compliance with the strictest regulatory environments;
|
| ● |
the Company will remain focused on providing premium cannabis products to medical patients in the jurisdictions in which the Company conducts business and any other jurisdiction in which the Company may conduct business in the future;
|
| ● |
the Company has the ability to amplify its commercial and brand power to become a global high-quality cannabis player;
|
| ● |
the Company will maintain its primary goal of sustainably increasing revenue in its core markets;
|
| ● |
the demand and momentum in the Company’s Israeli and Germany operations will be favourable to the Company;
|
| ● |
the Company will carry out its plans to position its brands as stated;
|
| ● |
the Company’s Company has the ability to realize upon the stated efficiencies and synergies the Company as a global organization with domestic expertise in Israel and Germany;
|
| ● |
providing a high-quality, reliable supply to the Company’s customers and patients will lead to recurring sales;
|
| ● |
the Company will introduce of new SKUs;
|
| ● |
the Company will realize the anticipated cost savings from the reorganization;
|
| ● |
the Company has the ability to achieve geographic diversification and brand recognition and the growth of the Company’s brands in the jurisdictions that the Company operates in or may expand to;
|
| ● |
the Company’s has the ability to address the ongoing needs and preferences of medical cannabis patients;
|
61
Management’s Discussion and Analysis
| ● |
the Company has the ability to realize upon its retail presence, distribution capabilities and data-driven insights;
|
| ● |
the future impact of the Regulations Amendment will be favourable to the Company;
|
| ● |
the Company will maintain its partnerships with third parties, suppliers and partners;
|
| ● |
the Company has the ability to achieve profitability in 2024;
|
| ● |
the accuracy of number of patients in Israel licensed by the MOH to consume medical cannabis;
|
| ● |
the accuracy of the number of patients paying out-of-pocket medical cannabis products in Germany;
|
| ● |
the anticipated decriminalization or legalization of adult-use recreational cannabis in Israel and Germany will occur;
|
| ● |
the Company has the ability to source premium and ultra-premium cannabis products exclusively and competition in this product segment;
|
| ● |
the anticipated impact of inflation and liquidity on the Company’s performance will be as forecasted;
|
| ● |
the accuracy with respect to the Company’s operating budget and the assumptions related thereto;
|
| ● |
the Company will remain as going concern;
|
| ● |
a favourable outcome with respect to the collection the payment awarded in the Judgment and the chances of the claim advancing or the potential outcome of the Test Kits Appeal;
|
| ● |
the Company’s Common Shares will remain listed on the Nasdaq and the CSE;
|
| ● |
the Company’s ability to maintain cannabis licensing in the jurisdictions in which the Company operates;
|
| ● |
the Company has the ability to obtain the renewal and/or extension of the Company’s licenses;
|
| ● |
the Company has the ability to meet operating cash requirements and future financing needs;
|
| ● |
the Company will meet or surpass its expectations regarding its revenue, expenses, profit margins and operations;
|
| ● |
the Company will meet or surpass its expectations regarding Gross Margins, EBITDA and Adjusted EBITDA from the Company’s operations;
|
| ● |
the Company will increase its revenue and margins in its Israeli medical cannabis market activities arising from its acquisitions;
|
| ● |
the Company has the ability to capitalize on future opportunities for the Company in Israel, particularly in the retail and distribution segments of the cannabis market;
|
| ● |
the Company will carry out its future expansion and growth opportunities for the Company in Germany and Europe and the timing of such;
|
| ● |
the Company will fulfill its contractual obligations and commitments; and
|
| ● |
the Company will complete the Proposed Transaction with Kadimastem.
|
Readers are cautioned that the above lists of forward-looking statements and assumptions are not exhaustive. Since forward-looking statements address future events and conditions, by their very nature they involve inherent risks and uncertainties. Actual results may differ materially from those currently anticipated or implied by such forward-looking statements due to a number of factors and risks. These include:
| ● |
the Company’s inability to achieve its business objectives and milestones under the stated timelines;
|
| ● |
the Company inability to carry out its business, strategies and operations;
|
62
Management’s Discussion and Analysis
| ● |
the Company’s inability to realize upon its intentions to expand the business, operations and potential activities of the Company;
|
| ● |
the Company will not expand its sales channels, distribution, delivery and storage capacity, and reach to medical cannabis patients;
|
| ● |
the competitive conditions of the cannabis industry and the growth of medical or adult-use recreational cannabis markets will be unfavourable to the Company in the jurisdictions in which the Company operates;
|
| ● |
the competitive conditions of the industry will be unfavourable to the Company, and the Company’s inability to maintain or grow its market share and maintain its competitive advantages;
|
| ● |
the Company will not commit to responsible growth and compliance with the strictest regulatory environments;
|
| ● |
the Company’s inability to remain focused on providing premium cannabis products to medical patients in the jurisdictions in which the Company conducts business and any other jurisdiction in which the Company may conduct business in the
future;
|
| ● |
the Company inability to amplify its commercial and brand power to become a global high-quality cannabis player;
|
| ● |
the Company will not maintain its primary goal of sustainably increasing revenue in its core markets;
|
| ● |
the demand and momentum in the Company’s Israeli and Germany operations will be unfavourable to the Company;
|
| ● |
the Company will not carry out its plans to position its brands as stated;
|
| ● |
the Company’s inability to realize upon the stated efficiencies and synergies of the Company as a global organization with domestic expertise in Israel and Germany;
|
| ● |
providing a high-quality, reliable supply to the Company’s customers and patients will not lead to recurring sales;
|
| ● |
the Company will not introduce of new SKUs;
|
| ● |
the Company’s inability to realize upon the anticipated cost savings from the reorganization;
|
| ● |
the Company’s inability to achieve geographic diversification and brand recognition and the growth of the Company’s brands in the jurisdictions that the Company operates in or may expand to;
|
| ● |
the Company’s inability to address the ongoing needs and preferences of medical cannabis patients;
|
| ● |
the Company’s inability to realize upon its retail presence, distribution capabilities and data-driven insights;
|
| ● |
the future impact of the Regulations Amendment will be unfavourable to the Company;
|
| ● |
the Company will not maintain its partnerships with third party suppliers and partners;
|
| ● |
the Company’s inability to achieve profitability in 2023;
|
| ● |
the inaccuracy of number of patients in Israel licensed by the MOH to consume medical cannabis;
|
| ● |
the inaccuracy of the number of patients paying out-of-pocket for medical cannabis products in Germany;
|
| ● |
the anticipated decriminalization or legalization of adult-use recreational cannabis in Israel and Germany will not occur;
|
| ● |
the Company’s ability to source premium and ultra-premium cannabis products exclusively and competition in this product segment;
|
| ● |
the anticipated impact of inflation and liquidity on the Company’s performance will not be as forecasted;
|
63
Management’s Discussion and Analysis
| ● |
the inaccuracy with respect to the Company’s operating budget and the assumptions related thereto;
|
| ● |
the Company will not remain as going concern;
|
| ● |
an unfavourable outcome of the negotiations or the Construction Proceedings;
|
| ● |
an unfavourable outcome with respect to the collection the payment awarded in the Judgment and the chances of the claim advancing or the potential outcome of the Appeal;
|
| ● |
the Company’s Common Shares will not remain listed on the Nasdaq and the CSE;
|
| ● |
the Company’s inability to maintain cannabis licensing in the jurisdictions in which the Company operates;
|
| ● |
the Company’s inability to obtain the renewal and/or extension of the Company’s licenses;
|
| ● |
the Company’s inability to meet operating cash requirements and future financing needs;
|
| ● |
the Company will not meet or surpass its expectations regarding its revenue, expenses, profit margins and operations;
|
| ● |
the Company will not meet or surpass its expectations regarding Gross Margins, EBITDA and Adjusted EBITDA from the Company’s operations;
|
| ● |
the Company will not increase its revenue and margins in its Israeli medical cannabis market activities arising from its acquisitions;
|
| ● |
the Company’s ability to capitalize on future opportunities for the Company in Israel, particularly in the retail and distribution segments of the cannabis market;
|
| ● |
the Company will not carry out its future expansion and growth opportunities for the Company in Germany and Europe and the timing of such; and
|
| ● |
the Company will not fulfill its contractual obligations and commitments; and
|
| ● |
the Company will not complete the Proposed Transaction with Kadimastem.
|
Readers are cautioned that the foregoing list of risk factors is not exhaustive. Additional information on these and other factors that could affect the business, operations or financial results of the Company are detailed under the headings “Risk and Factors” and “Contingent Liabilities and Commitments” of this MD&A. The Company and management caution readers not to place undue reliance on any forward-looking statements, which speak only as of the date made. Although the Company believes that the expectations reflected in the forward-looking statements are reasonable, it can give no assurance that such expectations will prove to have been correct. The Company and management assume no obligation to update or revise them to reflect new events or circumstances except as required by applicable securities laws.
Additional information about the assumptions, risks and uncertainties of the Company’s business and material factors or assumptions on which information contained in forward-looking statements is
based is provided in the Company’s disclosure materials, including in this MD&A under “Legal and Regulatory – Risk Factors” and the Company’s Annual Report under “Risk
Factors”, available on the Company’s profile on SEDAR+ at www.sedarplus.ca and on EDGAR at www.sec.gov/edgar.
All forward-looking statements in this MD&A is qualified by these cautionary statements.
ADDITIONAL INFORMATION
Additional information relating to the Company, including the Company’s Annual Report, is available on the Company’s profile on SEDAR+ at www.sedarplus.ca and on EDGAR at www.sec.gov/edgar.
- - - - - - - - - - - - - - - - - - - - - -
64