Exhibit 99.3

Management’s Discussion and Analysis
TABLE OF CONTENTS
2
Management’s Discussion and Analysis
INTRODUCTION
IM Cannabis Corp. (“IM Cannabis” or the “Company”) is a British
Columbia company operating in the international medical cannabis industry. The Company’s common shares (the “Common Shares”) trade under the ticker symbol “IMCC” on both the NASDAQ Capital Market (“NASDAQ”) and the Canadian Securities Exchange (“CSE”) as of March 1, 2021 and November 5, 2019, respectively.
This Management’s Discussion and Analysis (“MD&A”) reports on the consolidated financial condition and
operating results of IM Cannabis for the three and nine months ended September 30, 2024. Throughout this MD&A, unless otherwise specified, references to “we”, “us”, “our” or similar terms, as well as the “Company” and “IM Cannabis” refer to IM
Cannabis Corp., together with its subsidiaries, on a consolidated basis, and the “Group” refers to the Company, its subsidiaries, and Focus Medical Herbs Ltd.
This MD&A should be read in conjunction with the interim condensed consolidated financial statements of the Company and the notes thereto for the
three and nine months ended September 30, 2024 (the "Interim Financial Statements") and with the Company's audited annual consolidated financial statements and the notes thereto for the year ended December
31, 2023 (the “Annual Financial Statements”). References herein to “Q3 2024” and “Q3 2023” refer to the three and nine months ended September 30, 2024 and September 30, 2023, respectively, and references to
“2023” refer to the year ended December 31, 2023.
The Interim Financial Statements have been prepared by management in accordance with the International Financial Reporting Standards (“IFRS”) as issued by the International Accounting Standards Board (“IASB”). IFRS requires management to make certain judgments, estimates and assumptions that affect the
reported amount of assets and liabilities at the date of the Interim Financial Statements and the amount of revenue and expenses incurred during the reporting period. The results of operations for the periods reflected herein are not necessarily
indicative of results that may be expected for future periods. The Interim Financial Statements for the three and nine months ended September 30, 2024, include the accounts of the Group, which includes, among others, the following entities:
|
Legal Entity
|
Jurisdiction
|
Relationship with the Company
|
|
I.M.C. Holdings Ltd. (“IMC Holdings”)
|
Israel
|
Wholly-owned subsidiary
|
|
I.M.C. Pharma Ltd. (“IMC Pharma”)
|
Israel
|
Wholly-owned subsidiary of IMC Holdings
|
|
I.M.C. Farms Israel Ltd. (“IMC Farms”)
|
Israel
|
Wholly-owned subsidiary of IMC Holdings
|
|
Focus Medical Herbs Ltd. (“Focus”)
|
Israel
|
Subsidiary of IMC Holdings *
|
|
R.A. Yarok Pharm Ltd. (“Pharm Yarok”)
|
Israel
|
Wholly-owned subsidiary of IMC Holdings
|
|
Rosen High Way Ltd. (“Rosen High Way”)
|
Israel
|
Wholly-owned subsidiary of IMC Holdings
|
|
Revoly Trading and Marketing Ltd. dba Vironna Pharm (“Vironna”)
|
Israel
|
Subsidiary of IMC Holdings
|
|
Trichome Financial Corp. (“Trichome”)**
|
Canada
|
Wholly-owned subsidiary
|
* Effective February 26, 2024, IMC Holdings exercised its option to acquire a 74% ownership stake in Focus.
** Discontinued operations. For more information, please see the Company’s Annual Report, available on the Company’s profile on SEDAR+ at www.sedarplus.ca
and on EDGAR at www.sec.gov/edgar.
In this MD&A, unless otherwise indicated, all references: (i) “Company Subsidiaries” are to the Israeli
Subsidiaries and Adjupharm, (ii) “Israeli Operations” are to IMC Holdings and the Israeli Subsidiaries and (iii) “Trichome” are to Trichome Financial Corp. and its
subsidiaries.
3
Management’s Discussion and Analysis
All dollar figures in this MD&A are expressed in thousands of Canadian Dollars ($), except per share data and unless otherwise noted. All
references to “NIS” are to New Israeli Shekels. All references to “€” or to “Euros” are to Euros. All references to “US$” or to “U.S. Dollars” are to United States Dollars. The Company’s shares, options, units and warrants are not expressed in
thousands. Prices are not expressed in thousands.
NON-IFRS FINANCIAL MEASURES
Certain non-IFRS financial measures are referenced in this MD&A that do not have any standardized meaning under IFRS, including “Gross Margin”,
“EBITDA” and “Adjusted EBITDA”. The Company believes that these non-IFRS financial measures and operational performance measures, in addition to conventional measures prepared in accordance with IFRS, enable readers to evaluate the Company’s
operating results, underlying performance and prospects in a similar manner to the Company’s management. For a reconciliation of these non-IFRS financial measures to the most comparable IFRS financial measures, as applicable, see the “Metrics and Non-IFRS Financial Measures” section of the MD&A.
NOTE REGARDING THE COMPANY’S ACCOUNTING PRACTICES
The Company complies with IFRS 10 to consolidate the financial results of Focus, a holder of an Israeli Medical Cannabis Agency (the “IMCA”) license which allows it to import and supply cannabis products, on the basis of which IMC Holdings exercises “de facto control”. For a full explanation of the Company’s application of IFRS 10, see the
Company’s Annual Report, available on the Company’s profile on SEDAR+ at www.sedarplus.ca and on EDGAR at www.sec.gov/edgar. As of February 26, 2024, IMC Holdings holds 74% of Focus shares.
OVERVIEW – CURRENT OPERATIONS IN ISRAEL AND GERMANY
IM Cannabis is an international cannabis company that is focused on providing premium cannabis products to medical patients in Israel and Germany, two
prominent countries in the global medical cannabis industry. With the April 1st, 2024, partial cannabis legalization in Germany, the cannabis market is delivering
accelerated growth, especially within the medical sector as the barriers to entry for new patients have lowered. The trend is expected to continue as new users enter the market. IM Cannabis is shifting its focus and resources to concentrate on the
burgeoning German cannabis market where the Company is expected to drive accelerated growth. The Company leverages a transnational ecosystem powered by a unique data-driven approach and a globally sourced product supply chain. With an unwavering
commitment to responsible growth and compliance with the strictest regulatory environments, the Company strives to amplify its commercial and brand power to become a global high-quality cannabis player.
On November 7, 2022, the Company has exited its operations in Canada, deconsolidated Trichome pursuant to IFRS10 and announced that it is pivoting its
focus and resources to achieve sustainable and profitable growth in its highest value markets, Israel and Germany. For more information, please see the Company’s Annual Report, available on the Company’s profile on SEDAR+ at www.sedarplus.ca and on
EDGAR at www.sec.gov/edgar.
4
Management’s Discussion and Analysis
In the context of the deconsolidation of the Canadian operations, there are no remaining liabilities to the Company or any of its consolidated
subsidiaries related to the Canadian entities, except tax obligation of $839 related to debt settlement with L5 Capital Inc. (“L5 Capital”). The CCAA Proceedings were solely in respect of the Trichome Group.
As such, the Company’s other assets or subsidiaries, including those in Israel and Germany, were not parties to the CCAA Proceedings. Court materials filed in connection with Trichome's CCAA Proceedings can be found
at: https://www.ksvadvisory.com/insolvency-cases/case/trichome.
In Israel, the Company imports, distributes and sells cannabis to local medical patients by operating medical cannabis retail pharmacies, online
platforms, distribution center and logistical hubs operating through IMC Holdings’ subsidiaries, leveraging proprietary data and patient insights. The Company also preserves its existing proprietary genetics with third-party cultures facilities in
Israel.
In Germany, the IM Cannabis ecosystem operates through Adjupharm, importing and distributing cannabis to pharmacies for patients, and acting as the
Company’s entry point for potential Europe-wide distribution in the future.
With the recent regulatory changes in both Israel and in Germany, the market dynamics are changing.
Germany legalized cannabis on April 1, 2024, facilitating the access to medical cannabis prescriptions for patients and legalizing non-profit social
clubs starting July 1, 2024. The change in regulation has already led to rapid expansion within the last 6 months, driven by the number of new patients entering into the market, highlighting the importance of a stable supply chain able to respond
quickly to increases in demand. The Company’s German Operations Q3 revenue increased by about 200% between q1 and Q2, 67% increase from Q2 to Q3. Revenue of $1.15 million in Q1 increased to a revenue of $3.5M in Q2 and to a revenue of $5.85M in Q3.
The Company is focusing on increasing its supply to Germany to support further growth. The proposed Israeli medical cannabis regulatory reform entered into vigor on April 1, 2024, as well. The reform is also expected to facilitate the access to
medical cannabis for many new patient groups. While the impact in Germany was reflected immediately in the market, the Israeli reform is starting slowly and will take time for the impact to be reflected in the market.
For further information regarding the Germany new legislation and the Israeli Reform, please see sections "Regulatory Framework in Israel" and
" Regulatory Framework in Germany" below.
OUR GOAL – DRIVE PROFITABLE REVENUE GROWTH
Our primary goal is to sustainably increase revenue in each of our core markets, to accelerate our path to profitability and long-term shareholder
value while actively managing costs and margins.
HOW WE PLAN TO ACHIEVE OUR GOAL – CORE STRATEGIES
Our strategy of sustainable and profitable growth consists of:
| • |
Continue building on the increasing demand and positive momentum in Israel and Germany, supported by strategic alliances with Canadian suppliers and a highly skilled sourcing team, to cement its leadership position in markets where the
Company operates.
|
| • |
Develop and execute a long-term growth plan in Germany, based on the strong sourcing infrastructure in Israel which is powered by advanced product knowledge and regulatory expertise establishing, in the Company’s view, a competitive
advantage following the April 1, 2024, legalization in Germany.
|
5
Management’s Discussion and Analysis
| • |
Increasing inventory levels to meet the rising demand in Germany and securing new suppliers and additional supply chains from Israel and other countries to ensure product availability and support our growth in Germany.
|
| • |
Properly position brands with respect to target-market, price, potency and quality, such as our IMC brand in Israel and Germany.
|
| • |
Strong focus on efficiencies and synergies as a global organization with domestic expertise in Israel and Germany.
|
| • |
High-quality, reliable supply to our customers and patients, leading to recurring sales.
|
| • |
Ongoing introduction of new Stock Keeping Unit (“SKUs”) to keep consumers and patients engaged.
|
RESULTS – REVENUE GROWTH IN Q3 2024
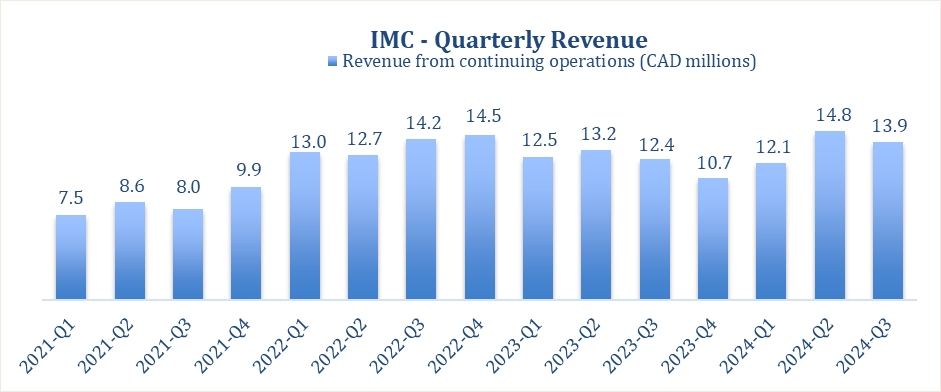
The Company operates in the Israeli and German medical cannabis markets. The Company was also actively servicing adult-use recreational consumers in
Canada; however these operations were discontinued and deconsolidated, effective November 7, 2022, pursuant to IFRS10. The Company announced that it is pivoting its focus and resources to achieve sustainable and profitable growth in its highest
value markets, Israel, Germany and Europe implementing a leaner organization strategy with the primary focus on achieving profitability in 2024.
Israel
In Israel, we continue to expand IMC brand recognition and supply the growing Israeli medical cannabis market with our branded products. The Company
offers medical cannabis patients a rich variety of high-end medical cannabis products through strategic alliances with Canadian suppliers supported by a highly skilled sourcing team. In addition to the benefits of the Group’s long-term presence in
Israel, we believe that with our strong sourcing infrastructure in Israel, and advanced product knowledge, regulatory expertise and strong commercial partnerships, the Company is well-positioned to address the ongoing needs and preferences of
medical cannabis patients in Israel and also to partially support the increased medical cannabis users needs in Germany.
The Company is also operating in the retail segment. The Company, through IMC Holdings, holds two licensed pharmacies, each selling medical cannabis
products to patients: (i) Vironna, a leading pharmacy in the Arab sector, and (ii) Pharm Yarok, the largest pharmacy in the Sharon plain area and the biggest call center in the country (Vironna and Pharm Yarok collectively, the “Israeli Pharmacies”).
6
Management’s Discussion and Analysis
The Company also operates a home-delivery services and an online retail platform, under the name “Pharm Yarok”,
which includes a customer service center and a licensed medical cannabis distribution center in Israel.
The operation in the retail segment in Israel positions IM Cannabis as a large distributor of medical cannabis in Israel. We are strategically focused
on establishing and reinforcing a direct connection with medical cannabis patients, providing direct access to IM Cannabis products, obtaining and leveraging market data and gaining a deeper understanding of consumer preferences. The operation of
the Israeli Pharmacies allows the Company to increase purchasing power with third-party product suppliers, offers potential synergies with our established call center and online operations, achieves higher margins on direct sales to patient and
creates the opportunity for up-sales across a growing range of products.
Germany
In Europe, the Company operates in Germany through Adjupharm, its German subsidiary and EU-GMP certified medical cannabis producer and distributor. We
continue to lay our foundation in Germany, which is currently the European market with a large number of medical cannabis patients. Leveraging our global supply chain, IM Cannabis continues to focus on growing its business in Germany to be
well-positioned through brand recognition in preparation for future regulatory reforms.
Similar to Israel, the Company’s focus in Germany is to import dried cannabis from its supply partners, which we believe will satisfy the rapid growth
in demand for high-THC cannabis across a variety of strains and qualities. In addition, Adjupharm sells cannabis extracts to meet the existing demand in the German market.
In the Company’s view, the strong sourcing infrastructure in Israel, powered by advanced product knowledge and regulatory expertise, will establish a
competitive advantage in Germany after the April 1, 2024, legalization. This is based on the premise that the German and Israeli markets share a number of common attributes such as robust commercial infrastructure, highly developed digital
capabilities, favourable demographics and customer preferences.
While the Company does not currently distribute products in other European countries, the Company intends to leverage the foundation established by
Adjupharm, its state-of-the-art warehouse and EU-GMP production facility in Germany (the “Logistics Center”), its vast knowledge in the cannabis market and costumers’ preferences and its network of
distribution partners to expand into other jurisdictions across the continent.
Adjupharm has an EU-GMP license that permits it to engage in additional production, cannabis testing and release activities. It allows Adjupharm to
repackage bulk cannabis, to perform stability studies and offer such services to third parties.
The IMC brand is well-known in the Israeli medical cannabis market, with reputable brands highly popular among Israeli consumers.
7
Management’s Discussion and Analysis
Israeli Medical Cannabis Business
The IMC brand has established its reputation in Israel for quality and consistency over the past 10 years and more recently with new high-end,
ultra-premium strains that have made it to the top-sellers list in pharmacies across the country.
The Group maintains a portfolio of strains sold under the IMC umbrella from which popular medical cannabis dried flowers and full-spectrum cannabis
extracts are produced.
The IMC brand offers four different product lines, leading with the Craft Collection which offers the highest quality Canadian craft cannabis flower
and has established IMC as the leader of the super-premium segment in Israel.
The Craft Collection – The IMC brand’s premium product line with indoor-grown, hand-dried and hand-trimmed
high-THC cannabis flowers. The Craft Collection includes exotic and unique cannabis strains such as Sup.S.

The Signature Collection – The IMC brand’s high-quality product line with greenhouse-grown or indoor grown,
high-THC cannabis flowers. The Signature Collection currently includes well known proprietary cannabis dried flowers such as Chemchew, FLO OG,, Roma , all an indoor-grown flowers.

8
Management’s Discussion and Analysis
The Full Spectrum Extracts – The IMC brand’s full spectrum, strain-specific cannabis extracts, includes
high-THC Roma®T20 oil.
The Company's Roma® product portfolio includes also oils. IMC’s Roma® strain is a high-THC medical cannabis
flower that offers a therapeutic continuum and is known for its strength and longevity of effect.
|
The WAGNERS™ brand launched in Israel in Q1 2022, with indoor-grown cannabis imported from Canada. The WAGNERS™ brand
was the first international premium, indoor-grown brand introduced to the Israel cannabis market, at a competitive price point. The WAGNERS™ brand includes Cherry Jam, Rainforest Crunch and Silverback#4.
 |
 |
|
BLKMKT™, the Company’s second Canadian brand, super-premium product line with indoor-grown,
hand-dried and hand-trimmed high-THC cannabis flowers. The BLKMKT™ includes BLK MLK, YA HEMI, PURPLE RAIN, JEALOUSY, Hemi GLTO and RAINBOW P.
|
|

LOT420 brand launched in Israel in Q2 2023, with super-premium
indoor-grown cannabis imported from Canada with high-THC. The LOT420 includes GLTO 33, Apps and Bans, O.C. The Company ceased from selling Atomic APP.

9
Management’s Discussion and Analysis

The PICO collection (minis)- Under the BLKMKT™ and LOT420 brands, the Company launched in 2023 a new type of
products (small flowers), a super-premium indoor-grown cannabis imported from Canada with high-THC. The Company launched in Q3 2024 new cannabis strain called Pico Rainbow P

Flower – The Company launched in Q2 2024 a new type of products, a super-premium indoor-grown cannabis imported
from Canada with high-THC. The Flower brand includes cannabis strain called California love and Face Sherb. In Q3 the Company re-launched California love and Face Sherb.

For more information, see “Strategy in Detail – Brands – New Product
Offerings” section of the MD&A.
10
Management’s Discussion and Analysis
German Medical Cannabis Business
In Germany, the company sells IMC-branded dried flower products and full spectrum extracts. The medical cannabis products are branded generically as
IMC to increase the brand awareness and build brand heritage among the German healthcare professionals.
After launching the first high THC strain in 2020, the portfolio has been carefully curated to include 8 high THC flowers, 1 high CBD flower, 1
balanced flower and 3 full spectrum extracts. In Q3, 2024 IMC expanded its portfolio, launching a new brand, "Selected" by IMC, with three high THC flowers: Jokerz, Gelato and Tropicana Banana.
The company is positioned among the top cannabis companies in Germany. The Group’s competitive advantage in Germany lies in its track record,
experience and brand reputation in Israel, as well as the proprietary data supporting the potential effectiveness of medical cannabis for the treatment of a variety of conditions.


Between our various geographies, the strategy for new products varies given that each market is at a different stage of development with respect to
regulatory regimes, patient and customer preferences and adoption rates.
Israel
In Q3 2024, the Company launched new cannabis strains in Israel, namely "PICO California #9" and "PICO Blk Mlk #10", "Pico Upside Down". In Q3 the
Company re-launched California and Face Sherb.
11
Management’s Discussion and Analysis
Israel
The company is concentrating on leveraging its skilled sourcing team and strategic alliances with Canadian suppliers as well as the import of medical
cannabis from its Canadian Facilities. The Company continues to import cannabis products and supply medical cannabis to patients through licensed pharmacies. To supplement growing demand, the Company continue its relationships with third-party
cultivation facilities in Israel for the propagation and cultivation of the Company’s existing proprietary genetics and for the development of new products.
In addition, the Company is operating through its subsidiaries who obtained a license from the IMCA to, among others, import cannabis products and
supply medical cannabis to patients.
Pursuant to the applicable Israeli cannabis regulations, following the import of medical cannabis, medical cannabis products are then packaged by
contracted GMP licensed producers of medical cannabis. The packaged medical cannabis products are then sold by the Group under the Company’s brands to local Israeli pharmacies directly or through contracted distributors.
Germany
The Company continues to expand its presence in the German market by forging partnerships with pharmacies and distributors across the country and
developing Adjupharm and its Logistics Center as the Company’s European hub. Adjupharm sources its supply of medical cannabis for the German market and from various EU-GMP certified European and Canadian suppliers. The Logistics Center is EU-GMP
certified, upgrading Adjupharm production technology and increasing its storage capacity to accommodate its anticipated growth. Adjupharm has a certification for primary repackaging, making it one of a handful of companies in Germany fully licenced
to repack bulk.
Adjupharm currently holds wholesale, narcotics handling, manufacturing, procurement, storage, distribution, and import/export licenses granted to it by
the applicable German regulatory authorities (the “Adjupharm Licenses”).
KEY HIGHLIGHTS FOR THE THIRD QUARTER OF 2024
In the third quarter of 2024, the Company continued to focus on its efforts and resources on growth in the Israeli and German cannabis markets with a
goal of reaching profitability. The Company’s key highlights and events for the third quarter ended September 30, 2024, include:
Short-term Loan Agreement
On July 1st, 2024, IMC Holdings entered into a short-term loan agreement with a non-financial institute in the amount of NIS 3,000 thousand
(approximately $1,113). Such loan bear interest at an annual rate of 12% and mature 62 days from the date of signing the loan agreement. IMC Holdings and the lender executed three amendments to the loan
agreement, each extending the maturity date by 30 days, thereby postponing the maturity date to November 30, 2023, under the same terms and conditions.
12
Management’s Discussion and Analysis
Consolidation 2024
On July 5, 2024, the Company announced that the board of directors of the Company (the "Board") has approved a
consolidation of its issued and outstanding common shares ("Common Shares") based on one post-consolidated Common Share for every six pre-consolidated Common Shares (the "Consolidation"). The
Board has set July 12, 2024, as the effective date of the Consolidation and anticipates the Common Shares to trade on a post-consolidated basis effective July 12, 2024, subject to final confirmation from the Canadian Securities Exchange (the "CSE") and Nasdaq Stock Market LLC (the "NASDAQ"). The exercise price and/or conversion price and number of Common Shares issuable under any of the Company's outstanding
convertible securities were proportionately adjusted in connection with the Consolidation.
Shareholders of record as of the effective date received a letter of transmittal from Computershare Investor Services Inc., the Company's registrar and
transfer agent for the Common Shares, providing instructions for the exchange of their Common Shares as soon as practicable following the effective date. Registered shareholders may also obtain a copy of the letter of transmittal by accessing the
Company's SEDAR+ profile at www.sedarplus.ca. Until surrendered, each share certificate or direct registration system statement representing pre-consolidated Common Shares will represent the number of whole post-consolidated Common Shares to which
the holder is entitled as a result of the Consolidation. No action was required by beneficial holders to receive post-consolidation Common Shares in connection with the Consolidation. Beneficial holders who hold their Common Shares through
intermediaries (e.g., a broker, bank, trust company investment dealer or other financial institution) and who have questions regarding how the Consolidation will be processed should contact their intermediaries with respect to the Consolidation.
On July 12, 2024, the Company announced that, further to its press release dated July 5, 2024, effective July 12, 2024, the Company's common shares ("Common Shares") are trading on the CSE and NASDAQ on a 6:1 post-consolidated basis (the "Consolidation"). The Company's trading symbol remains "IMCC" on both the CSE and NASDAQ. The Company's new CUSIP and ISIN
numbers are 44969Q406 and CA44969Q4060, respectively. After giving effect to the Consolidation, the Common Shares were reduced from 13,394,136 to 2,232,359 Common Shares. No fractional Common Shares were issued in connection with the Consolidation.
Instead, all fractional Common Shares equal to or greater than one-half resulting from the Consolidation were rounded to the next whole number, otherwise, the fractional Common Share were cancelled. The exercise price and/or conversion price and
number of Common Shares issuable under any of the Company's outstanding convertible securities were proportionately adjusted in connection with the Consolidation. Computershare Investor Services Inc., the Company's registrar and transfer agent for
the Common Shares, has mailed letters of transmittal to registered shareholders of record as of July 12, 2024, providing instructions for the exchange of their Common Shares as soon as practicable following the effective date. Registered
shareholders may also obtain a copy of the letter of transmittal by accessing the Company's SEDAR+ profile at www.sedarplus.ca. Until surrendered, each Common Share certificate or direct registration system statement representing pre-consolidated
Common Shares will represent the number of whole post-consolidated Common Shares to which the holder is entitled as a result of the Consolidation. No action is required by beneficial holders to receive post-consolidation Common Shares in connection
with the Consolidation. Beneficial holders who hold their Common Shares through intermediaries (e.g., a broker, bank, trust company investment dealer or other financial institution) and who have questions regarding how the Consolidation will be
processed should contact their intermediaries with respect to the Consolidation.
*After giving effect to the Consolidation, the Common Shares were reduced from 13,394,136 to 2,232,359 (after rounding fractional Common Shares).
13
Management’s Discussion and Analysis
NASDAQ Notification of Regaining Compliance
On July 29, 2024, the Company announced that on July 26, 2024, it has received formal notice from The Nasdaq Stock Market, LLC ("Nasdaq") stating that the Company has regained compliance with the minimum bid price requirement set forth in Rule 5550(a)(2) of the Nasdaq Listing Rules (the "Minimum Bid Price
Requirement"). IMC is now in compliance with all applicable listing standards and will continue to be listed and traded on the NASDAQ Stock Market.
As previously announced, the Company was notified by Nasdaq on August 1, 2023, that it was not in compliance with the Minimum Bid Price of $1.00 per
share for 30 consecutive business days as required by the Listing Rules of Nasdaq and on January 31, 2024, has received a 180-calendar day extension, until July 29, 2024, from Nasdaq to regain compliance. Since then, Nasdaq staff has determined
that for the last 10 consecutive business days, from July 12, 2024, to July 25, 2024, the closing bid price of the Company's Ordinary Shares has been at $1.00 per share or greater. Accordingly, the Company has regained compliance with Listing Rule
5550(a)(2) and the matter has been closed.
Payment schedule with third party
On July 30, 2024, the Company entered into an acknowledgment and payment schedule agreement with a third party regarding unpaid fees, charges, and
disbursements for services rendered to the Company. According to the terms of the agreement, the Company shall pay the sum of $54,000 on the first business day of each month for a period of twenty-four (24) months, with the first payment due on
November 1, 2024.
Changes Regarding the New Mizrahi Facility
On August 1, 2024, the credit line of approximately $1,850 related to the New Mizrahi Facility, as defined herein, was converted into a six-month
short-term loan, bearing an annual variable interest rate of P+1.9% (with the Israel Prime interest rate as of the submission date being 6%). For more information, please see " LIQUIDITY AND CAPITAL RESOURCES” below.
Appointment of Shmulik Arbel to Board of Directors
On September 11, 2024, the Company announced that Mr. Shmulik Arbel has been appointed to the Company’s Board of Directors (the "Board") effective September 9, 2024. Mr. Arbel brings a wealth of experience in strategic plans that drive profitability, as well as, finance and corporate governance, further strengthening the company's
commitment to driving growth while focusing on sustainable profitability.
Mr. Arbel retired as Deputy CEO from Leumi, Israel's largest banking group, in April 2023, where he was instrumental in business growth and leading the
service revolution. With over 25 years of experience at Leumi, Arbel has held senior roles throughout the organization, such as head of retail banking, head of the corporate division, and as chairman of Leumi UK. With key roles in Israel, New York
and London, Mr. Arbel has a wide view on international business.
SUBSEQUENT EVENTS
Adjupharm's Preliminary Q3, 2024 Performance
On October 2, 2024, the Company announced that the preliminary sales results in Germany by its Adjupharm, for the third quarter of 2024 have
significantly exceeded expectations, showing a 50% increase in revenue compared to the second quarter, where Adjupharm sold about CAD$ 3.5M.
Since the partial legalization of cannabis in Germany came into effect in April 2024, the demand for cannabis products in pharmacies has increased
significantly, emphasizing the importance of a robust, reliable supply chain.
* For more information about the actual results for Germany, see below the “OPERATIONAL RESULTS” section of
the MD&A.
14
Management’s Discussion and Analysis
Non-brokered Private Placement of Units
On October 4, 2024, the Company announced its intention to complete a non-brokered private placement offering of up to US$1,613,000 through the sale of
up to 760,406 units (each, an “Unit”) at a price per Unit (the “Offering Price”) calculated on the basis of the deemed price per common shares in the capital of the
Company (each, a “Share”) equal to the 10-day volume weighted average price of the Shares on Canadian Securities Exchange (the “Exchange”) ending on the trading day
preceding October 3, 2024.
Each Unit will be comprised of one Share and one Share purchase warrant (each, a “Warrant”). Each Warrant shall
entitle the holder thereof to acquire one additional Share (each, a “Warrant Share") at a price of $4.32, equal to a 50% premium to the Offering Price (the “Warrant Exercise
Price”) at any time prior to 5:00 p.m. (Toronto time) on second anniversary of the closing date. The offering Price is C$2.88 per Unit.
On November 12, further to its press release dated October 4, 2024 (the “October 4 Release”), the Company has closed its previously announced non-brokered private placement offering (the “Offering”) effective November 12, 2024 (the “Closing Date”) through the issuance
of 742,517 Units for gross proceed of C$2,138,448.96 Capitalized terms not otherwise defined herein have the meanings attributed to them in the October 4 Release.
Each Unit was sold at a price of C$2.88 per Unit, calculated on the basis of the deemed price per Share equal to the 10-day volume weighted average
price of the Shares on the Exchange ending on the trading day preceding October 3, 2024, and consisted of one Share and one Warrant.
All securities issued under the Offering are subject to: (i) a four month and one day hold period from the date of issuance and (ii) applicable legends as required pursuant
to the United States Securities Act of 1933, as amended.
The Company intends to use the proceeds from the Offering for the repayment of a loan to A.D.I. CAR ALARMS & STEREO SYSTEMS Ltd. provided to the
Company’s subsidiary IMC Holdings Ltd. on October 11, 2022.
Debt Settlement and Loan Bonus
Since October 2022, the Company, through its subsidiaries, has borrowed from various groups more than US$8,000,000 (together, the “Loans”). As required by the lenders, Mr. Oren Shuster, the Company's CEO and chairman of the Board has personally guaranteed the Loans. The
independent members of the board of directors commissioned a valuation to determine the value of Mr. Shuster’s personal guarantees, which ascribes the benefit to the Company to be approximately US$560,000, approximately C&758,240.00, based on
an exchange rate of US$1.00 = C$1.354 as at October 3, 2024, as published on the website of the Bank of Canada (the “Benefit”). The Company and
Mr. Shuster entered into a settlement agreement to settle the amount of a pre-funded Share purchase warrant (a “Pre-Funded Warrant”), at the
Offering Price. Each Pre-Funded Warrant entitles the holder to purchase one Settlement Share for a price of $0.00001, upon receipt of shareholder approval to allow Mr. Shuster to become a control person (as defined in the policies of the
Exchange).
15
Management’s Discussion and Analysis
On November 12, 2024, the Company also announced that the Company has completed a debt settlement (the “Debt Settlement” and together, with the Offering, the “Transactions”)
in the amount of US$560,000 approximately C&758,240.00, based on an exchange rate of US$1.00 = C$1.354 as at October 3, 2024, as published on the website of the Bank of Canada with Oren Shuster, the Company’s Chief Executive Officer, in
connection with the Benefit, to preserve the Company’s cash for working capital through the issuance of 110,576 Settlement Shares and 152,701 Pre-Funded Warrants at a deemed price of C$2.88.
Each Pre-Funded Warrant will entitle the holder to purchase one Settlement Share for a price of $0.00001, upon receipt of shareholder approval to allow
Mr. Shuster to become a control person (as defined in the policies of the Exchange).
All securities issued in consideration for the Benefit are subject to: (i) a four month and one day hold period from the date of issuance and (ii)
applicable legends as required pursuant to the United States Securities Act of 1933, as amended.
Oren Shuster, a director and officer of the Company, Shmulik Arbel, a director of the Company and Rafael Gabay, an insider of the
Company, (together, the “Participating Insiders”) each participated in the Offering and Mr. Shuster participated in the Debt Settlement. Mr.
Shuster acquired 194,110 Units, 110,576 Settlement Shares and 152,701 Pre-Funded Warrants, Mr. Arbel acquired 48,349 Units and Mr. Gabay acquired 194,088 Units.
The participation of the Participating Insiders in the Offering constitutes a “related party transaction”, as such term is defined in MI 61-101 and
would require the Company to receive minority shareholder approval for and obtain a formal valuation for the subject matter of, the transaction in accordance with MI 61-101, prior to the completion of such transaction. However, in completing the
Offering, the Company has relied on exemptions from the formal valuation and minority shareholder approval requirements of MI 61-101, on the basis of subsections 5.5(g) and 5.7(g) – Financial Hardship of MI
61-101, as the Company is (i) in a situation of serious financial difficulty; (ii) the Transactions are designed to improve the financial position of the Company as (x) the Company would be unable to repay back the loan provided to A.D.I. CAR
ALARMS & STEREO SYSTEMS Ltd. provided to the Company’s subsidiary IMC Holdings Ltd. without the completion of the Offering and (y) would have been unable to obtain Loans without Mr. Shuster personal guaranteeing them; (iii) the circumstances
described in Section 5.5(f) of MI 61-101 are not applicable, and (iv) the Board and independent directors (as such term is defined in MI 61-101) have, acting in good faith, determined that (i) and (ii) apply and the terms of the Transactions are
reasonable in the circumstances of the Company.
The Transactions were approved by the members of the Board who are independent for the purposes of the Transactions, respectively. No special committee
was established in connection with the Transactions; however, the independent members of the Board commissioned a third-party valuator to determine the Benefit.
Further details will be included in a material change report to be filed by the Company. The Company did not file a material change report more than 21
days before the closing date of the Transactions as the participation of Participating Insiders in the Offering was not definitively known to the Corporation until closing. In the Company’s view, the shorter period was necessary to permit the
Company to close the Transactions in a timeframe consistent with usual market practice for transactions of this nature and was reasonable and necessary to improve the Company’s financial position.
Option and Warrant Cancellation
The Company cancelled an aggregate of 31,305 (“Options”) to purchase Shares, which were previously granted to
board members, officers, employees, advisors and consultants of the Issuer (each a “Participant”). Management reviewed the Issuer’s outstanding Options and determined that certain Options granted to such
Participants, at exercise prices ranging from $6.60 to $600 per Share, no longer represented a realistic incentive to motivate such Participants.
16
Management’s Discussion and Analysis
The Company cancelled an aggregate of 142,784 Share purchase warrants (the “Subject Warrants”) to purchase
Shares, which were previously granted to Mr. Shuster. Management reviewed the Issuer’s outstanding warrants and determined that the Subject Warrants at an exercise price of US$9.00 per Share, no longer represented a realistic incentive to motivate
Mr. Shuster.
For more information, please refer to the press release dated October 4, 2024.
Option Grants
The Company approved the grant of 31,305 options to certain eligible persons of the Company, at an exercise price of US$2.24 per Share, with an expiry
date of two years from the date of issuance (the “Option Grants”). The Options Grants vest as follows: one third vest immediately, one third vests on the six-month anniversary and the final one third vests on
the twelve-month anniversary.
For more information, please refer to the press releases dated October 4, 2024 and November 12, 2024.
Oren Shuster, a director and officer of the Company, Shmulik Arbel, a director of the Company and Rafael Gabay, an insider of the
Company, (together, the “Participating Insiders”) each participated in the Offering and Mr. Shuster participated in the Debt Settlement. Mr.
Shuster acquired 194,110 Units, 110,576 Shares and 152,701 Pre-Funded Warrants, Mr. Arbel acquired 48,349 Units and Mr. Gabay 194,088 Units.
17
Management’s Discussion and Analysis
FINANCIAL HIGHLIGHTS
Below is the analysis of the changes that occurred for the three and nine months ended September 30, 2024, with further commentary provided below.
|
|
For the period ended
September 30,
|
For the three months ended
September 30,
|
For the year ended December 31,
|
|||||||||||||||||
|
Financial Results
|
2024
|
2023
|
2024
|
2023
|
2023
|
|||||||||||||||
|
Net Revenues
|
$
|
40,696
|
$
|
38,106
|
$
|
13,883
|
$
|
12,370
|
$
|
48,804
|
||||||||||
|
Gross profit before fair value impacts in cost of sales
|
$
|
5,818
|
$
|
9,715
|
$
|
3,170
|
$
|
2,738
|
$
|
10,830
|
||||||||||
|
Gross margin before fair value impacts in cost of sales (%)
|
14
|
%
|
25
|
%
|
23
|
%
|
22
|
%
|
22
|
%
|
||||||||||
|
Operating Income (Loss)
|
$
|
(9,452
|
)
|
$
|
(7,627
|
)
|
$
|
(953
|
)
|
$
|
(2,259
|
)
|
$
|
(12,792
|
)
|
|||||
|
Net Income (Loss)
|
$
|
(10,558
|
)
|
$
|
(6,708
|
)
|
$
|
(1,082
|
)
|
$
|
(2,136
|
)
|
$
|
(10,228
|
)
|
|||||
|
Loss per share attributable to equity holders of the Company - Basic (in CAD) *
|
$
|
(4.29
|
)
|
$
|
(2.95
|
)
|
$
|
(0.41
|
)
|
$
|
(0.96
|
)
|
$
|
(0.74
|
)
|
|||||
|
Loss per share attributable to equity holders of the Company - Diluted (in CAD) *
|
$
|
(4.29
|
)
|
$
|
(2.95
|
)
|
$
|
(0.41
|
)
|
$
|
(0.96
|
)
|
$
|
(0.74
|
)
|
|||||
* Shares Consolidation - On July 12, 2024, the Company consolidated its issued and outstanding common shares based on one post-consolidated Common
Share for every six pre-consolidated Common Shares. Post Consolidation, total Common Shares were reduced from 13,394,136 to 2,232,359 Common Shares (after rounding fractional Common Shares).
|
For the Nine Months Ended
September 30,
|
For the Three months ended
September 30,
|
For the Year ended December 31,
|
||||||||||||||||||
|
2024
|
2023
|
2024
|
2023
|
2023
|
||||||||||||||||
|
Average net selling price of dried flower (per Gram)
|
$
|
6.01
|
$
|
5.34
|
$
|
6.20
|
$
|
4.35
|
$
|
5.14
|
||||||||||
|
Quantity of dried flower sold (in Kilograms)
|
6,408
|
6,528
|
2,202
|
2,558
|
8,609
|
|||||||||||||||
The Overview of Financial Performance includes reference to “Gross Margin”, which is a non-IFRS financial measure that the Company defines as the
difference between revenue and cost of revenues divided by revenue (expressed as a percentage), prior to the effect of a fair value adjustment for inventory and biological assets. For more information on non-IFRS financial measures, see the “Non-IFRS Financial Measures” and “Metrics and Non-IFRS Financial Measures” sections of the MD&A.
OPERATIONAL RESULTS
In each of the markets in which the Company operates, the Company must navigate evolving customer and patient trends in order to continue to be
competitive with other suppliers of medical cannabis products.
18
Management’s Discussion and Analysis
The Company believes that there are several key factors creating tailwinds to facilitate further industry growth. In Israel, the number of licensed
medical patients currently stands at 115,072 as of October 2024. This figure is expected to grow in the coming years and may further benefit from regulatory change liberalizing the cannabis market in Israel. IM Cannabis is a large distributor of
medical cannabis in Israel.
Up until April 2024, The German medical cannabis market has been slower over the past few years to develop, mainly due to the difficulty in medical
patients accessing prescriptions and insurance reimbursements. Starting April 2024 after the legalization was officially approved by the Bundestag (Germany Parliament) on April 1st, 2024, The Company which has already seen an increase in the number of patients paying out-of-pocket for medical cannabis products in Germany during the past few years, is experiencing and expecting a significant increase of
demand which leads to increase in the Revenue.
|
Germany Region Revenue for the Three months ended
|
||||||||||||
|
September 30, 2024
|
June 30,
2024
|
March 31,
2024
|
||||||||||
|
Revenue for the period
|
$
|
5,817
|
$
|
3,508
|
$
|
1,152
|
||||||
|
Q vs Q Increase %
|
66
|
%
|
205
|
%
|
-
|
|||||||
The Germany market demands for the Company products is high and the Company is investing efforts on building a strong high volume supply chain that
will allow the Company to continue its present and its growth in the region.
19
Management’s Discussion and Analysis
REVENUES AND GROSS MARGINS
REVENUES
The revenues of the Group are primarily generated from sales of medical cannabis products to customers in Israel and Germany. The reportable
geographical segments in which the Company operates are Israel and Germany.
For the nine months ended September 30:
|
Israel
|
Germany
|
Adjustments
|
Total
|
|||||||||||||||||||||||||||||
|
2024
|
2023(*)
|
|
2024
|
2023(*)
|
|
2024
|
2023(*)
|
|
2024
|
2023(*)
|
|
|||||||||||||||||||||
|
Revenues
|
$
|
30,219
|
$
|
33,941
|
$
|
10,477
|
$
|
4,165
|
$
|
-
|
$
|
-
|
$
|
40,696
|
$
|
38,106
|
||||||||||||||||
|
Segment income (loss)
|
$
|
(8,261
|
)
|
$
|
(2,974
|
)
|
$
|
993
|
$
|
(1,035
|
)
|
$
|
-
|
$
|
-
|
$
|
(7,268
|
)
|
$
|
(4,009
|
)
|
|||||||||||
|
Unallocated corporate expenses
|
$
|
-
|
$
|
-
|
$
|
-
|
$
|
-
|
$
|
(2,184
|
)
|
$
|
(3,618
|
)
|
$
|
(2,184
|
)
|
$
|
(3,618
|
)
|
||||||||||||
|
Total operating (loss)
|
$
|
(8,261
|
)
|
$
|
(2,974
|
)
|
$
|
993
|
$
|
(1,035
|
)
|
$
|
(2,184
|
)
|
$
|
(3,168
|
)
|
$
|
(9,452
|
)
|
$
|
(7,627
|
)
|
|||||||||
|
Depreciation& amortization
|
$
|
1,520
|
$
|
2,139
|
$
|
122
|
$
|
126
|
$
|
-
|
$
|
-
|
$
|
1,642
|
$
|
2,265
|
||||||||||||||||
* See Note 1 under “Review of Financial Performance – Financial Highlights” section of the MD&A.
The consolidated revenues of the Group for the nine months ended September 30, 2024, were attributed to the sale of medical cannabis products in Israel
and Germany.
| ● |
Revenues for the nine months ended September 30, 2024, and 2023 were $40,696 and $38,106, respectively, representing an increase of $2,590 or 7%. The increase is mainly attributed to accelerated growth in Germany revenue of $6,312 and
decreased Revenue in Israel of $3,722 net. The decrease in Israel is attributed to Oranim deal cancellation resulted with decreased Revenue of approximately $5,099 compared to the nine months ended September 2023.
Revenues for the three months ended September 30, 2024, and 2023 were $13,883 and $12,370, respectively, representing an increase of $1,513 or 12%. The increase is
mainly attributed to accelerated growth in Germany revenue of $4,279 and decreased Revenue in Israel of $2,766 net. The decrease in Israel is attributed to Oranim deal cancellation resulted with decreased Revenue of $3,166 compared to
the three months ended September 2023.
|
| ● |
Revenues from the Israeli operation was attributed to the sale of medical cannabis through the Company’s subsidiaries and the revenues from the Israeli Pharmacies the Company owns, mostly from cannabis products.
|
| ● |
In Germany, Company revenues were attributed to the sale of medical cannabis through Adjupharm.
|
20
Management’s Discussion and Analysis
Total dried flower sold for the nine months ended September 30, 2024, was 6,408kg at an average selling price of $6.01 per gram compared to 6,528kg for
the same period in 2023 at an average selling price of $5.34 per gram, mainly attributed to the inventory life cycle, products diversity, discounts given and increased competition in the region. For the nine months ended September 30, 2024,
Germany’s share of total revenue has significantly increased compared to the corresponding period in 2023. This increase has had a considerable impact, reflected in a higher average price due to favorable market conditions and growing demand.
Together, these factors have contributed to an overall positive effect on our revenue performance.
COST OF REVENUES
Cost of revenues is comprised of purchase of raw materials and finished goods, import costs, production costs, product laboratory testing, shipping and
salary expenses. Inventory is later expensed to the cost of sales when sold. Direct production costs are expensed through the cost of sales.
The cost of revenues for the nine months ended September 30, 2024 and 2023 were $34,878 and $28,391, respectively, representing an increase of $6,487
or 23%. This is mainly due to an increase in material costs of approximately $7,098 of which; clearing old raw materials of approximately $940, accrued for slow inventory of approximately $2,199 and increased inventory sales resulted with an
increase of approximately $3,959 which is offset by reduced in other costs net of approximately $606.
The cost of revenues for the three months ended September 30, 2024 and 2023 were $10,713 and $9,632, respectively, representing an increase of $1,081
or 11%. This is mainly due to an increase costs of approximately $1,195 including an accrual of $600 for slow inventory, and is offset by decrease in other costs net of approximately $100.
GROSS PROFIT
Gross profit for the nine months ended September 30, 2024, and 2023 was $5,771 and $9,005, respectively, representing a decrease of $3,234 or 36%.
Gross profit for the three months ended September 30, 2024, and 2023 was $3,148 and $2,645, respectively, representing an increase of $503 or 19%.
Gross profit included losses from realized fair value adjustments on inventory sold of $(47) and $(710) for the nine months ended September 30, 2024,
and 2023, respectively.
EXPENSES
GENERAL AND ADMINISTRATIVE
General and administrative expenses for the nine months ended September 30, 2024, and 2023 were $6,846 and $7,708, respectively, representing a
decrease of $862 or 11%. General and administrative expenses for the three months ended September 30, 2024, and 2023 were $2,351 and $2,145, respectively, representing an increase of $206 or 10%.
The general and administrative expenses are comprised mainly from salaries to employees in the amount of $1,616 and $524 for the nine and three months
ended September 30, 2024, professional fees in the amount of $2,325 and $901 for the nine and three months ended September 30, 2024, depreciation and amortization in the amount of $400 and $140 for the nine and three months ended September 30,
2024, insurance costs in the amount of $992 and $335 for the nine and three months ended September 30, 2024 and Other expenses in the amounts of $1,513 and $451 for the nine and three months ended September 30, 2024.
21
Management’s Discussion and Analysis
SELLING AND MARKETING
Selling and marketing expenses for the nine months ended September 30, 2024, and 2023 were $5,279 and $7,991, respectively, representing a decrease of
$2,712 or 34%. Selling and marketing expenses for the three months ended September 30, 2024, and 2023 were $1,506 and $2,564, respectively, representing a decrease of $1,058 or 41%.
The decrease in the selling and marketing expenses for the nine and three months ended September 30, 2024, is mainly attributed to Oranim revoke
agreement of approximately $1,334 and $747, respectively, and decrease of $1,378 and $311, respectively, in Selling and marketing expenses.
PROVISION FOR REVOKING ORANIM TRANSACTION
Due to the revocation of the Oranim agreement on April 15, 2024, the Company accrued $2,753 other operating expenses in Q1 2024 for losses expected on
Q2 2024 due to the clearing of Oranim assets and liabilities from the consolidated balances. The total expense for the nine months ended September 30, 2024, is $2,734.
SHARE-BASED COMPENSATION
Share-based compensation expense for the nine months ended September 30, 2024, and 2023 was $364 and $316, respectively, representing an increase of
$48 or 15%.
For the three months ended September 30, 2024, and 2023 share-based compensation expense from continuing operations was $244 and $195, respectively,
representing an increase of $49 or 25%.
OPERATING EXPENSE RATIO
The operating expense ratio for the 9 months ended Sep 30, 2024, was 31% excluding the one-time expense outcome of Oranim deal cancellation vs. 44% for the 9 months ended
Sep 30, 2023, representing an increased efficiency of about 30%.
The operating expense ratio for the 3 months ended Sep 30, 2024, was 30% vs. 40% for the 3 months ended Sep 30, 2023, representing an increased
efficiency of about 25%.
The efficiency ratio improvement is resulting from decreased operational costs and increased revenue.
FINANCING
Financing income (expense) net, for the nine months ended September 30, 2024, and 2023 was $(2,082) and $869, respectively, representing a decrease of
$2,951 or 340%. For the three months ended September 30, 2024, and 2023 financing income (expense), net, was $(155) and $248, respectively, representing a decrease of $403 or 163%.
22
Management’s Discussion and Analysis
NET INCOME/LOSS
Net loss for the nine months ended September 30, 2024, and 2023 was $10,558 and $6,708, respectively, representing a net loss decrease of $3,850 or
57%. For the three months ended September 30, 2024, and 2023 net loss was $1,082 and $2,136, respectively, representing a net loss decrease of $1,054 or 49%. The net loss decreases related to factors impacting net income described above.
NET INCOME (LOSS) PER SHARE BASIC AND DILUTED
Basic loss per share is calculated by dividing the net profit attributable to holders of Common Shares by the weighted average number of Common Shares
outstanding during the period. Diluted profit per Common Share is calculated by adjusting the earnings and number of Common Shares for the effects of dilutive warrants and other potentially dilutive securities. The weighted average number of Common
Shares used as the denominator in calculating diluted profit per Common Share excludes unissued Common Shares related to Options as they are antidilutive.
Basic Income (Loss) per Common Share for the nine months ended September 30, 2024, and 2023 were $(4.29) and $(2.95) per Common Share, respectively.
For the three months ended September 30, 2024, and 2023 basic Loss per Common Share from continuing operations were $(0.41) and $(0.96) per Common Share, respectively.
Diluted net loss per share for the nine months ended September 30, 2024, and 2023 is $(4.29) and $(2.95) respectively and $(0.41) and $(0.96) for the
three months ended September 30, 2024, respectively.
* Shares Consolidation - On July 12, 2024, the Company consolidated its issued and outstanding common shares based on one post-consolidated Common
Share for every six pre-consolidated Common Shares. Post Consolidation, total Common Shares were reduced from 13,394,136 to 2,232,359 Common Shares (after rounding fractional Common Shares).
TOTAL ASSETS
Total assets as of September 30, 2024, were $44,635, compared to $48,813 as at December 31, 2023, representing a decrease of $4,178 or 9%. The decrease
is mainly attributed to the Oranim agreement cancelation at total amount of $9,494, of which mainly attributed to goodwill at total amount of $3,499, intangible asset in the amount of $1,414, Inventory in the amount of $837, Trade receivables in
the amount of $1,324, Property plant and equipment in the amount of $783 and reduction of Cash and cash equivalents in the amount of $346. In addition to the Oranim revocation agreement affect, there is a total asset increase of $5,316 mainly due
to an increase of $8,084 in trade receivables and $491 increase of Cash and cash equivalents, offset by $4,829 reduction in Inventory and $936 reduction of intangible assets.
TOTAL LIABILITIES
Total liabilities on September 30, 2024, were $40,425 compared to $35,113 at December 31, 2023, representing an increase of $5,312 or 15%. The change
was mainly due to the Oranim agreement cancelation of $6,771 of which mainly attributed to a decrease in PUT option liability in the amount of $1,973 and a decrease in purchase consideration payable in the amount of $2,172, a decrease in trade
payables in the amount of $1,597, a decrease of $176 in other accounts payable, a decrease of $372 in lease liabilities and a decrease of $326 in deferred tax liability.
In addition to the Oranim revocation agreement affect, there is a total liability increase of $12.1 million mainly due to increase of $8.9 million in
trade payables offset by a $1.6 million reduction in other accounts payable.
23
Management’s Discussion and Analysis
LIQUIDITY AND CAPITAL RESOURCES
For the nine months ended September 30, 2024, the Company recorded revenues of $40,696.
The Company can face liquidity fluctuations from time to time, resulting from delays in sales and slow inventory movements.
In January 2022, Focus entered a revolving credit facility with an Israeli bank, Bank Mizrahi (the “Mizrahi Facility”).
The Mizrahi Facility is guaranteed by Focus assets. Advances from the Mizrahi Facility is being used for working capital needs. The Mizrahi Facility has a total commitment of up to NIS 15 million (approximately $6,000) and has a one-year term for
on-going needs and 6 months term for imports and purchases needs. The Mizrahi Facility is renewable upon mutual agreement by the parties. The borrowing base is available for draw at any time throughout the Mizrahi Facility and is subject to several
covenants to be measured on a quarterly basis (the “Mizrahi Facility Covenants”).
The Mizrahi Facility bears interest at the Israeli Prime interest rate plus 1.5%.
On May 17, 2023, the Company and Bank Mizrahi entered to new credit facility with total commitment of up to NIS 10,000 (approximately $3,600) (the “New Mizrahi Facility”). The New Mizrahi Facility consists of NIS 5,000 credit line and NIS 5,000 loan to be settled with 24 monthly installments from May 17, 2023. This loan bears interest at the Israeli Prime
interest rate plus 2.9%. As of September 30, 2024 Focus has drawn down $2,623 in respect of the new Mizrahi facility (comprised of approx. $1,826 credit line and $797 loan). The New Credit facility is also subject to several covenants to be
measured on a quarterly basis which are not met as of September 30, 2024, therefore the loan is classified as short-term loan. On August 1, 2024, the credit line of approximately NIS 1,825 related to the New Mizrahi Facility was converted into a
six-month short-term loan, bearing an annual variable interest rate of P+1.9% (with the Israel Prime interest rate as of the submission date being 6%)
The Company's CEO and Chairman, provided to the bank a personal guarantee in the amount of the outstanding borrowed amount, allowing the New Mizrahi
Facility to remain effective.
On November 12, 2024, the Company announced that further to its press release dated October 4, 2024 (the “October 4 Release”), the Company has closed its previously announced non-brokered private placement offering (the “Offering”) effective November 12, 2024 (the “Closing Date”) through the issuance of 742,517 Units for gross proceed of C$2,138,448.96 Capitalized terms not otherwise defined herein have the meanings attributed to
them in the October 4 Release.
Each Unit was sold at a price of C$2.88 per Unit, calculated based on the deemed price per Share equal to the 10-day volume weighted average price of
the Shares on the Exchange ending on the trading day preceding October 3, 2024, and consisted of one Share and one Warrant.
Each Warrant entitles the holder thereof to acquire one Warrant Share at a price of C$4.32 per Warrant Share, calculated as a 50% premium to the
Offering Price, at any time prior to 5:00 pm (Toronto Time) on the date that is twenty-four months following the Closing Date.
All securities issued under the Offering are subject to: (i) a four month and one day hold period from the date of issuance and (ii) applicable legends as required pursuant
to the United States Securities Act of 1933, as amended.
24
Management’s Discussion and Analysis
The Company intends to use the proceeds from the Offering for the repayment of a loan to A.D.I. CAR ALARMS & STEREO SYSTEMS Ltd. provided to the
Company’s subsidiary IMC Holdings Ltd. on October 11, 2022.
The Company also announced that the Company has completed a debt settlement (the “Debt Settlement”) in the amount of US$560,000 approximately C&758,240.00, based on an exchange rate of US$1.00 = C$1.354 as at October 3, 2024, as published on the website of the Bank of
Canada with Oren Shuster, the Company’s Chief Executive Officer, in connection with the Benefit, to preserve the Company’s cash for working capital through the issuance of Shares and Pre-Funded Warrants at a deemed price of C$2.88.
Each Pre-Funded Warrant will entitle the holder to purchase one Settlement Share for a price of $0.00001, upon receipt of shareholder approval to allow
Mr. Shuster to become a control person (as defined in the policies of the Exchange).
All securities issued in consideration for the Benefit are subject to: (i) a four month and one day hold period from the date of issuance and (ii)
applicable legends as required pursuant to the United States Securities Act of 1933, as amended.
Oren Shuster, a director and officer of the Company, Shmulik Arbel, a director of the Company and Rafael Gabay, an insider of the
Company, (together, the “Participating Insiders”) each participated in the Offering and Mr. Shuster participated in the Debt Settlement. Mr.
Shuster acquired 194,110 Units, 110,576 Shares and 152,701 Pre-Funded Warrants, Mr. Arbel acquired 48,349 Units and Mr. Gabay 194,088 Units.
The participation of the Participating Insiders in the Offering constitutes a “related party transaction”, as such term is defined in MI 61-101 and
would require the Company to receive minority shareholder approval for and obtain a formal valuation for the subject matter of, the transaction in accordance with MI 61-101, prior to the completion of such transaction. However, in completing the
Offering, the Company has relied on exemptions from the formal valuation and minority shareholder approval requirements of MI 61-101, on the basis of subsections 5.5(g) and 5.7(g) – Financial Hardship of MI 61-101, as the Company is (i) in a
situation of serious financial difficulty; (ii) the Transactions are designed to improve the financial position of the Company as (x) the Company would be unable to repay back the loan provided to A.D.I. CAR ALARMS & STEREO SYSTEMS Ltd.
provided to the Company’s subsidiary IMC Holdings Ltd. without the completion of the Offering and (y) would have been unable to obtain Loans without Mr. Shuster personal guaranteeing them; (iii) the circumstances described in Section 5.5(f) of MI
61-101 are not applicable, and (iv) the Board and independent directors (as such term is defined in MI 61-101) have, acting in good faith, determined that (i) and (ii) apply and the terms of the Transactions are reasonable in the circumstances of
the Company.
The Transactions were approved by the members of the Board who are independent for the purposes of the Transactions, respectively. No special committee
was established in connection with the Transactions; however, the independent members of the Board commissioned a third-party valuator to determine the Benefit.
Further details will be included in a material change report to be filed by the Company. The Company did not file a material change report more than 21
days before the closing date of the Transactions as the participation of Participating Insiders in the Offering was not definitively known to the Corporation until closing. In the Company’s view, the shorter period was necessary to permit the
Company to close the Transactions in a timeframe consistent with usual market practice for transactions of this nature and was reasonable and necessary to improve the Company’s financial position.
25
Management’s Discussion and Analysis
As of September 30, 2024, the Group's cash and cash equivalents totaled $1,958 and the Group's working capital deficit (current assets minus current
liabilities) amounted to ($11,605). In the nine months ending September 30, 2024, the Group had an operating loss of ($9,452) and cash flows from operating activities of $3,122.
As of September 30, 2024, the Group’s financial liabilities consisted of accounts payable which have contractual maturity dates within one year. The
Group manages its liquidity risk by reviewing its capital requirements on an ongoing basis. Based on the Group’s working capital position on September 30, 2024, management considers liquidity risk to be high.
As of September 30, 2024, the Group has identified the following liquidity risks related to financial liabilities (undiscounted):
|
Less than one year
|
1 to 5 years
|
6 to 10 years
|
> 10 years
|
|||||||||||||
|
Contractual Obligations
|
$
|
12,955
|
$
|
685
|
-
|
-
|
||||||||||
The maturity profile of the Company’s other financial liabilities (trade payables, other account payable and accrued expenses, and warrants) as of
September 30, 2024, are less than one year.
|
Payments Due by Period
|
||||||||||||||||||||
|
Contractual Obligations
|
Total
|
Less than one year
|
1 to 3 years
|
4 to 5 years
|
After 5 years
|
|||||||||||||||
|
Debt
|
$
|
13,084
|
$
|
12,679
|
$
|
405
|
$
|
-
|
$
|
-
|
||||||||||
|
Finance Lease Obligations
|
$
|
556
|
$
|
276
|
$
|
280
|
$
|
-
|
$
|
-
|
||||||||||
|
Total Contractual Obligations
|
$
|
13,640
|
$
|
12,955
|
$
|
685
|
$
|
-
|
$
|
-
|
||||||||||
The Group’s current operating budget includes various assumptions concerning the level and timing of cash receipts from sales and cash outflows for
operating expenses and capital expenditures, including cost saving plans. In 2023 The Company’s board of directors approved a cost saving plan, to allow the Company to continue its operations and meet its cash obligations. The cost saving plan
entailed reducing costs through efficiencies and synergies primarily involving the following measures: discontinuing loss-making activities, reducing payroll and headcount, reduction in compensation paid to key management personnel (including
layoffs of key executives), operational efficiencies and reduced capital expenditures. These actions are resulting in cost savings during 2024, and the company will continue its efforts for efficiency operations.
Despite the cost savings plan and restructuring as described above, the projected cash flow for 2024 indicates that it is uncertain that the Group will
generate sufficient funds to continue its operations and meet its obligations as they become due. The Group continues to evaluate additional sources of capital and financing. However, there is no assurance that additional capital and or financing
will be available to the Group, and even if available, whether it will be on terms acceptable to the Group or in amounts required.
These conditions raise substantial doubt about the Company’s ability to continue as a going concern. The consolidated financial statements do not
include any adjustments relating to the recoverability and classification of assets or liabilities that might be necessary should the Company be unable to continue as a going concern. The Interim Financial Statements have been prepared on the basis
of accounting principles applicable to a going concern, which assumes that the Company will continue in operation for the foreseeable future and will be able to realize its assets and discharge its liabilities in the normal course of operations.
The Interim Financial Statements do not include any adjustments to the amounts and classification of assets and liabilities that would be necessary should the Company be unable to continue as a going concern. Such adjustments could be material.
26
Management’s Discussion and Analysis
SHARE CAPITAL
The Company’s authorized share capital as of September 30, 2024, consist of an unlimited number of Common shares without par value of 2,232,359,
following the July 12th, 2024, Consolidation (after rounding fractional Common Shares). The Common Shares confer upon their holders the right to participate in the
general meeting with each Common Share carrying the right to one vote on all matters. The Common Shares also allow holders to receive dividends if declared and to participate in the distribution of surplus assets in the case of liquidation of the
Company.
OTHER SECURITIES
As of September 30, 2024, the Company also has the following outstanding securities which are convertible into, or exercisable or exchangeable for,
voting or equity securities of the Company: 32,982 Options, 3,044 2019 Broker Compensation Options (as defined below), 49,057 Offered Warrants (as defined below), 961,603 from 2023 LIFE Offering Warrants and 410,192 Debentures.
FINANCIAL BACKGROUND
On October 11, 2019, the Company completed the Reverse Takeover Transaction, effected by way of a “triangular merger” between the Company, IMC Holdings
and a wholly owned subsidiary of the Company pursuant to Israeli statutory law.
In connection with the Reverse Takeover Transaction, the Company completed a private placement offering of 19,460,527 subscription receipts (each a “Subscription Receipt”) on a pre-2021 Share Consolidation basis (as defined below) of a wholly owned subsidiary of the Company at a price of $1.05 per Subscription Receipt for aggregate gross proceeds of $20,433.
Upon completion of the Reverse Takeover Transaction, each Subscription Receipt was exchanged for one unit comprised of one (1) common share and one-half of one (1/2) warrant (each whole warrant, a “2019 Listed
Warrant”). Each 2019 Listed Warrant was exercisable for one Common Share at an exercise price of $1.30 until October 11, 2021. A total of 9,730,258 2019 Listed Warrants were issued and listed for trading on the CSE under the ticker
“IMCC.WT”. The 2019 Listed Warrants expired on October 11, 2021.
The Company also issued to the agent who acted on its behalf in connection with the Reverse Takeover Transaction, a total of 1,199,326 2019 Broker
Compensation Options (the “2019 Broker Compensation Options”). Following the 2021 Share Consolidation, the 2019 Broker Compensation Options were adjusted to require four 2019 Broker Compensation Options to be
exercised for one underlying unit at an adjusted exercise price of $4.20, with each unit exercisable into one Common Share and one-half of one Common Share purchase warrant (the “2019 Unlisted Warrants”).
Following the 2021 Share Consolidation, the 2019 Unlisted Warrants were adjusted to require four 2019 Unlisted Warrants to be exercised for one Common Share at an adjusted exercise price of $5.20. The 2019 Broker Compensation Options and the 2019
Unlisted Warrants expired on August 2022.
27
Management’s Discussion and Analysis
On February 12, 2021, the Company consolidated all its issued and outstanding Common Shares on the basis of one (1) post-consolidation Common Share for
each four (4) pre-consolidation Common Shares (the “2021 Share Consolidation”) to meet the NASDAQ minimum share price requirement.
On November 17, 2022, the Company completed a second share consolidation (the “2022 Share Consolidation”) by
consolidating all its issued and outstanding Common Shares based on one (1) post-Consolidation Common Share for each ten (10) pre-Consolidation Common Shares.
On May 7, 2021, the Company completed an offering (the “2021 Offering”) for a total of 6,086,956 Common Shares
and 3,043,478 Common Share purchase warrants (the “2021 Offered Warrants”). Following the 2022 Share Consolidation, the 2021 Offered Warrant were adjusted to require the (10) 2021 Offered Warrant to be
exercised for one (1) Common Share at an adjusted exercise price of US$72 for a term of 5 years from the date of closing of the 2021 Offering.
The Company also issued a total of 182,609 broker compensation options (the “2021 Broker Compensation Options”)
to the agents who acted on its behalf in connection with the 2021 Offering. Following the 2022 Share Consolidation, the 2021 Broker Compensation Option were adjusted to require the (10) 2021 Broker Compensation Options for one (1) Common Share at
an adjusted exercise price of US$66.1, at any time following November 5, 2021, until November 5, 2022. There are 182,609 2021 Broker Compensation Options outstanding.
In January and February of 2023, the Company issued an aggregate of 2,828,248 units of the Company (each a “Life Unit”)
at a price of US$1.25 per Life Unit for aggregate gross proceeds of US$3,535 in a series of closings pursuant to a non-brokered private placement offering to purchasers resident in Canada (except the Province of Quebec) and/or other qualifying
jurisdictions relying on the listed issuer financing exempt under Part 5A of National Instrument 45-106 – Prospectus Exemptions (the “LIFE Offering”). Each Life Unit consisted of one Common Share and one
Common Share purchase warrant (each a “Life Warrant”), with each Life Warrant entitling the holder thereof to purchase one additional Common Share at an exercise price of US$1.50 for a period of 36 months
from the date of issue.
In addition, a non-independent director of the Company subscribed for an aggregate of 131,700 Life Units under the LIFE Offering at an aggregate
subscription price of US$165. The director’s subscription price was satisfied by the settlement of US$165 in debt owed by the Company to the director certain consulting services previously rendered by the director to the Company.
In connection with the LIFE Offering, the Company and Odyssey Trust Company entered a series of warrant indentures on January 30, 2023 (the “First LIFE Warrant Indenture”), February 7, 2023 (the “Second LIFE Warrant Indenture”) and February 16, 2024 (the “Third LIFE Warrant
Indenture”) to govern the terms and conditions of the Life Warrants.
Concurrent with the LIFE Offering, the Company issued an aggregate of 2,317,171 units on a non-brokered private placement basis at a price of US$1.25
per unit for aggregate gross proceeds of US$2,897 (the “Concurrent Offering”). The Concurrent Offering was led by insiders of the Company. The units offered under the Concurrent Offering were sold under
similar terms as the Life Offering and were offered for sale to purchasers in all provinces and territories of Canada and jurisdictions outside Canada pursuant to available prospectus exemptions other than for the LIFE Offering exemption. All units
issued under the Concurrent Offering were subject to a statutory hold period of four months and one day in accordance with applicable Canadian securities laws.
28
Management’s Discussion and Analysis
On July 12, 2024, the Company closed a non-brokered private placement (the “Offering”) of secured convertible
debentures of the Company (each, a “Debenture”) for aggregate proceeds of $2,091,977. The Debentures were issued to holders of short-term loans and obligations owed by the Company or its wholly owned
subsidiaries. The Debentures will mature on May 26, 2025, and will not incur interest except in the event of default. The Debentures may be converted into common shares of the Company (each, a “Share”) at a
conversion price of $0.85 per Share.
As of September 30, 2024, and December 31, 2023, there were 1,010,660 warrants outstanding (following 2024 Share Consolidation, as defined below),
re-measured by the Company, using the Black-Scholes pricing model, in the amount of $13 and $38, respectively. For the nine months ended September 30, 2024, and 2023, the Company recognized a revaluation gain (loss) in the consolidated statement of
profit or loss and other comprehensive income, of $24 and $(4,547), respectively, in which the unrealized gain is included in finance income (expense).
On July 12, 2024, the Company consolidated all its issued and outstanding Common Shares based on one (1) post-consolidation Common Share for each Six
(6) pre-consolidation Common Shares (the “2024 Share Consolidation”) to meet the NASDAQ minimum share price requirement.
On November 12, 2024, the Company closed its
previously announced non-brokered private placement offering (the “Offering”) effective November 12, 2024 (the “Closing Date”) through the issuance of 742,517 Units for gross proceed of C$2,138,48.96 Capitalized terms not otherwise defined herein have the meanings attributed to them in the October 4 Release.
Each Unit was sold at a price of C$2.88 per Unit, calculated based on the deemed price per Share equal to the 10-day volume weighted average price of
the Shares on the Exchange ending on the trading day preceding October 3, 2024, and consisted of one Share and one Warrant.
Each Warrant entitles the holder thereof to acquire one Warrant Share at a price of C$4.32 per Warrant Share, calculated as a 50% premium to the
Offering Price, at any time prior to 5:00 pm (Toronto Time) on the date that is twenty-four months following the Closing Date.
The Company also announced that the Company has completed a debt settlement (the “Debt Settlement” and together, with the Offering, the “Transactions”) in the
amount of US$560,000 approximately C&758,240.00, based on an exchange rate of US$1.00 = C$1.354 as at October 3, 2024, as published on the website of the Bank of Canada with Oren Shuster, the Company’s Chief Executive Officer, in connection
with the Benefit, to preserve the Company’s cash for working capital through the issuance of 110,576 Settlement Shares and 152,701 Pre-Funded Warrants at a deemed price of C$2.88.
29
Management’s Discussion and Analysis
OPERATING, FINANCING AND INVESTING ACTIVITIES
The following table highlights the Company’s cash flow activities for the nine and three months ended September 30, 2024, and 2023:
|
For the Nine Months Ended
September 30,
|
For the Three months ended
September 30,
|
For the Year ended December 31,
|
||||||||||||||||||
|
Net cash provided by (used in):
|
2024
|
2023
|
2024
|
2023
|
2023
|
|||||||||||||||
|
Operating activities
|
$
|
3,122
|
$
|
(7,857
|
)
|
$
|
2,754
|
$
|
5,355
|
$
|
(8,075
|
)
|
||||||||
|
Investing activities
|
$
|
(472
|
)
|
$
|
(553
|
)
|
$
|
(74
|
)
|
$
|
-
|
$
|
(1,182
|
)
|
||||||
|
Financing activities
|
$
|
(22
|
)
|
$
|
9,454
|
$
|
(665
|
)
|
$
|
(1,223
|
)
|
$
|
9,417
|
|||||||
|
Effect of foreign exchange
|
$
|
(2,483
|
)
|
$
|
(2,189
|
)
|
$
|
(757
|
)
|
$
|
(4,149
|
)
|
$
|
(796
|
)
|
|||||
|
Increase (Decrease) in cash
|
$
|
145
|
$
|
(1,145
|
)
|
$
|
1,258
|
$
|
(17
|
)
|
$
|
(636
|
)
|
|||||||
Operating activities provided cash of $3,122 and used cash of $7,857 for the nine months ended September 30, 2024, and 2023, respectively. Operating
activities provided cash of $2,754 and $5,355 for the three months ended September 30, 2024, and 2023, respectively. This variance is primarily due to business activities of the Company including corporate expenses for salaries, professional fees,
and marketing expenses in Israel and Germany out of which a $2,764 and $nil increase is attributed to Loss from deconsolidation of Oranim for the nine months ended September 30, 2024, and 2023, respectively.
Investing activities used cash of $(472) and $(553) for the nine months ended September 30, 2024, and 2023, respectively. Investing activities used
cash of $(74) and $nil for the three months ended September 30, 2024, and 2023, respectively, of which a decease of $(346) and $nil is attributed to Deconsolidation of Oranim for the nine months ended September 30, 2024, and 2023, respectively.
Financing activities used cash of $(22) and provided cash of $9,454 for the nine months ended September 30, 2024, and 2023, respectively. Financing
activities used cash of $665 and $1,223 for the three months ended September 30, 2024, and 2023, respectively. The decrease for the nine months is primarily due to the reduction of proceeds from issuance of warrants and share capital by $6,585 and
$1,688 respectively, an increase of repayment of bank loan and credit facilities in the amount of $4,427 and set-off by an increase in proceeds from loans in the amount of $2,912.
SELECTED ANNUAL INFORMATION – CONTINUING OPERATIONS
|
For the year ended
|
December 31,
2023 |
December 31, 2022
|
December 31, 2021
|
|||||||||
|
Revenues
|
$
|
48,804
|
$
|
54,335
|
$
|
34,053
|
||||||
|
Net Loss
|
$
|
(10,228
|
)
|
$
|
(24,922
|
)
|
$
|
(664
|
)
|
|||
|
Basic net income (Loss) per share:
|
$
|
(4.45
|
)
|
$
|
(18.81
|
)
|
$
|
0.12
|
||||
|
Diluted net income (Loss) per share:
|
$
|
(4.45
|
)
|
$
|
(22.87
|
)
|
$
|
(21.72
|
)
|
|||
|
Total assets
|
$
|
48,813
|
$
|
60,676
|
$
|
129,066
|
||||||
|
Total non-crurent liabilities
|
$
|
2,305
|
$
|
3,060
|
$
|
21,354
|
||||||
30
Management’s Discussion and Analysis
SUMMARY OF INTERIM RESULTS
|
For the three months ended
|
September 30,
2024 |
June 30,
2024 |
March 31,
2024 |
December 31, 2023
|
||||||||||||
|
Revenues
|
$
|
13,883
|
$
|
14,750
|
$
|
12,063
|
$
|
10,698
|
||||||||
|
Net Loss
|
$
|
(1,082
|
)
|
$
|
(3,456
|
)
|
$
|
(6,020
|
)
|
$
|
(3,520
|
)
|
||||
|
Basic net income (Loss) per share:
|
$
|
(0.41
|
)
|
$
|
(1.36
|
)
|
$
|
(2.52
|
)
|
$
|
(1.47
|
)
|
||||
|
Diluted net loss per share:
|
$
|
(0.41
|
)
|
$
|
(1.36
|
)
|
$
|
(2.52
|
)
|
$
|
(1.47
|
)
|
||||
|
For the three months ended
|
September 30, 2023
|
June 30,
2023 |
March 31,
2023 (1)
|
December 31, 2022
|
||||||||||||
|
Revenues
|
$
|
12,370
|
$
|
13,207
|
$
|
12,529
|
$
|
14,461
|
||||||||
|
Net income (Loss)
|
$
|
(2,136
|
)
|
$
|
(3,706
|
)
|
$
|
(866
|
)
|
$
|
(9,650
|
)
|
||||
|
Basic net income (Loss) per share:
|
$
|
(0.96
|
)
|
$
|
(1.57
|
)
|
$
|
(0.3
|
)
|
$
|
(7.94
|
)
|
||||
|
Diluted net income (Loss) per share:
|
$
|
(0.96
|
)
|
$
|
(1.57
|
)
|
$
|
(0.3
|
)
|
$
|
(7.70
|
)
|
||||
Note 1 - The figures disclosed here for the three months ended March 31, 2023, encompass updates and adjustments made during Q2 2023 to the Company’s
previously filed unaudited interim financial statements. The adjustments and updates were immaterial.
* Shares Consolidation - On July 12, 2024, the Company consolidated its issued and outstanding common shares based on one post-consolidated Common
Share for every six pre-consolidated Common Shares. Post Consolidation, total Common Shares were reduced from 13,394,136 to 2,232,359 Common Shares (after rounding fractional Common Shares).
METRICS AND NON-IFRS FINANCIAL MEASURES
This MD&A refers to “Gross Margin”, “EBITDA”, and “Adjusted EBITDA”. These financial measures are not recognized measures under IFRS and do not
have a standardized meaning prescribed by IFRS and are therefore unlikely to be comparable to similar measures presented by other companies. Rather, these measures are provided as additional information to complement those IFRS measures by
providing further understanding of our results of operations from management’s perspective. Accordingly, these measures should neither be considered in isolation nor as a substitute for analysis of our financial information reported under IFRS.
Management defines Gross Margin as the difference between revenue and cost of goods sold divided by revenue (expressed as a percentage), prior to the
effect of a fair value adjustment for inventory and biological assets. Management defines EBITDA as income earned or lost from operations, as reported, before interest, tax, depreciation and amortization.
Adjusted EBITDA is defined as EBITDA, adjusted by removing other non-recurring or non-cash items, including the unrealized change in fair value of
biological assets, realized fair value adjustments on inventory sold in the period, share-based compensation expenses, and revaluation adjustments of financial assets and liabilities measured on a fair value basis. Management believes that Adjusted
EBITDA is a useful financial metric to assess its operating performance on a cash adjusted basis before the impact of non-recurring or non-cash items. The closest IFRS metric to EBITDA and Adjusted EBITDA is “operating loss”.
31
Management’s Discussion and Analysis
The non-IFRS financial measures can provide investors with supplemental measures of our operating performance and thus highlight trends in our core
business that may not otherwise be apparent when relying solely on IFRS measures. We also believe that securities analysts, investors and other interested parties frequently use non-IFRS financial measures in the evaluation of issuers. These
financial measures are metrics that have been adjusted from the IFRS statements in an effort to provide readers with a normalized metric in making comparisons more meaningful across the cannabis industry. However, other companies in our industry
may calculate this measure differently, limiting their usefulness as comparative measures.
Our management also uses these non-IFRS financial measures to facilitate operating performance comparisons from period to period, to prepare annual
operating budgets and forecasts and to determine components of management compensation. As required by Canadian securities laws, we reconcile these non-IFRS financial measures to the most comparable IFRS measures.
GROSS MARGIN
|
For the nine Months Ended
September 30,
|
For the three months ended
September 30,
|
For the Year ended December 31,
|
||||||||||||||||||
|
2024
|
2023
|
2024
|
2023
|
2023
|
||||||||||||||||
|
Net Revenue
|
$
|
40,696
|
$
|
38,106
|
$
|
13,883
|
$
|
12,370
|
$
|
48,404
|
||||||||||
|
Cost of sales
|
$
|
(34,878
|
)
|
$
|
(28,391
|
)
|
$
|
(10,713
|
)
|
$
|
(9,632
|
)
|
$
|
(37,974
|
)
|
|||||
|
Gross profit before FV adjustments
|
$
|
5,818
|
$
|
9,715
|
$
|
3,170
|
$
|
2,738
|
$
|
10,830
|
||||||||||
|
Gross margin before FV adjustments (non-IFRS)
|
14
|
%
|
25
|
%
|
23
|
%
|
22
|
%
|
22
|
%
|
||||||||||
* See Note 1 under “Review of Financial Performance – Financial Highlights” section of the MD&A.
32
Management’s Discussion and Analysis
EBITDA AND ADJUSTED EBITDA
|
For the Nine Months ended
September 30,
|
For the Three Months ended
September 30,
|
For the Year ended December 31,
|
||||||||||||||||||
|
2024
|
2023
|
2024
|
2023
|
2023
|
||||||||||||||||
|
Operating Loss
|
$
|
(9,452
|
)
|
$
|
(7,627
|
)
|
$
|
(953
|
)
|
$
|
(2,259
|
)
|
$
|
(12,792
|
)
|
|||||
|
Depreciation & Amortization
|
$
|
1,642
|
$
|
2,265
|
$
|
451
|
$
|
678
|
$
|
2,996
|
||||||||||
|
EBITDA
|
$
|
(7,810
|
)
|
$
|
(5,362
|
)
|
$
|
(502
|
)
|
$
|
(1,581
|
)
|
$
|
(9,796
|
)
|
|||||
|
IFRS Biological assets fair value adjustments, net1
|
$
|
47
|
$
|
710
|
$
|
22
|
$
|
93
|
$
|
984
|
||||||||||
|
Share-based payments
|
$
|
364
|
$
|
316
|
$
|
244
|
$
|
195
|
$
|
225
|
||||||||||
|
Restructuring cost 2
|
$
|
-
|
$
|
617
|
$
|
-
|
$
|
-
|
$
|
617
|
||||||||||
|
Other non-recurring costs 3
|
$
|
2,734
|
$
|
-
|
$
|
-
|
$
|
-
|
$
|
-
|
||||||||||
|
Adjusted EBITDA (Non-IFRS)
|
$
|
(4,665
|
)
|
$
|
(3,719
|
)
|
$
|
(236
|
)
|
$
|
(1,293
|
)
|
$
|
(7,970
|
)
|
|||||
* See Note 1 under “Review of Financial Performance – Financial Highlights” section of the MD&A.
Notes:
| 1. |
Losses from unrealized change in fair value of biological assets and realized fair value adjustments on inventory. See “Cost of Revenues” section of the MD&A.
|
| 2. |
Costs attributable to the Israel Restructuring and closure of Sde Avraham Farm in 2022, and to Israel reorganization plan of the company’s management and operations in 2023.
|
| 3. |
Due to revocation of the Oranim transaction dated April 16, 2024.
|
The Company’s Adjusted EBITDA loss for the nine months ended September 30, 2024, was increased by $946 vs. the nine months ended September 30, 2023,
mainly due to the Inventory clearance process and slow inventory accrual during year 2024 sums up to approximately $3,140.
The Company’s Adjusted EBITDA loss for the three months ended September 30, 2024, was decreased by $1,057 vs. the nine months ended September 30, 2023,
and is including a $600 accrual for slow inventory.
CONTINGENT LIABILITIES AND COMMITMENTS
RENTAL LIABILITIES
The table below summarizes the maturity profile of the Group’s lease liabilities based on contractual undiscounted payments (including interest payments):
September 30, 2024:
|
Less than one year
|
1 to 5 years
|
6 to 10 years
|
>10 years
|
|||||||||||||
|
Lease liabilities
|
$
|
276
|
$
|
280
|
-
|
-
|
||||||||||
December 31, 2023:
|
Less than one year
|
1 to 5 years
|
6 to 10 years
|
>10 years
|
|||||||||||||
|
Lease liabilities
|
$
|
466
|
$
|
818
|
-
|
-
|
||||||||||
33
Management’s Discussion and Analysis
LITIGATION AND REGULATORY PROCEEDING
COVID-19 TEST KITS CLAIM, DISTRICT COURT OF STUTTGART
On November 19, 2021, Adjupharm filed a statement of claim (the “Claim”) to the District Court of Stuttgart
(the “Stuttgart Court”) against Stroakmont & Atton Trading GmbH (“Stroakmont & Atton”), its shareholders and managing
directors regarding a debt owed by Stroakmont & Atton to Adjupharm in an amount of approximately EUR 947,563 for COVID-19 test kits purchased by Stroakmont & Atton from Adjupharm at the end of March 2021. In January 2022, Stroakmont &
Atton filed its statement of defence to the Stuttgart Court in which they mainly stated two arguments for their defense:
| 1. |
The contractual party of the Company was not the Stroakmont & Atton. The contract with Stroakmont & Atton was only concluded as a sham transaction to cover up a contract with a company named Uniclaro GmbH. Therefore, Stroakmont
& Atton is not the real purchaser rather than Uniclaro GmbH.
|
| 2. |
The Company allegedly placed an order with Uniclaro GmbH for a total of 4.3 million Clongene COVID-19 tests, of which Uniclaro GmbH claims to have a payment claim against the Company for a partial delivery of 380,400 Clongene COVID-19
tests in the total amount of EUR 941,897.20. Uniclaro GmbH has assigned this alleged claim against the Company to Stroakmont & Atton Trading GmbH, and Stroakmont & Atton Trading GmbH has precautionary declared a set-off against the
Company’s claim.
|
On March 22, 2022, Adjupharm filed a response to Stroakmont & Atton’s statement of defence and rejected both allegations with a variety of legal
arguments and facts and also offered evidence to the contrary in the form of testimony from the witnesses in question.
The burden of proof for the allegation that the contract with Stroakmont & Atton was only concluded as a sham transaction lies with the opponents,
and they offered evidences to the court in the form of testimony from certain witnesses.
A court hearing with witnesses was held on January 11, 2023, and on February 22, 2023, where witnesses testified.
According to the court the witnesses were not able to provide required evidence for the allegation regarding the sham transaction
with Stroakmont & Atton. On April 5, 2023, Stuttgart Court announced its decision (the "Judgment") and sentenced Stroakmont & Atton to
pay to Adjupharm EUR 947,563.68 plus interest in the amount of 5 percentage points above the German basis rate since May 8, 2021. In addition, Stroakmont & Atton was sentenced to pay Adjupharm EUR 6,551.20 plus interest at 5 percentage points above the German basis rate since December 14, 2021.
34
Management’s Discussion and Analysis
The directors of Stroakmont, Mr. Simic and Mr. Lapeschi, were not sentenced and in this respect, the claim was dismissed against them regarding their
personal liability. Adjupharm shall pay 2/3 of the Stuttgart Court expenses and the out-of-court expenses of Mr. Simic and Mr. Lapeschi. Stroakmont shall bear 1/3 of the Stuttgart Court expenses and 1/3 of the out-of-court expenses of Adjupharm.
The remaining out-of-court expenses shall be borne by each party.
Furthermore, the court did not decide on the counterclaims from an alleged order by Adjupharm for 4.3 million Clongene tests due to a set-off
prohibition. This set-off prohibition follows from a jurisdiction agreement concluded between Adjupharm and Uniclaro, which determined the courts in Hamburg to be the competent court to decide about such allegations.
The Judgment is not yet final and, therefore, cannot be enforced. On May 5, 2023, Adjupharm and Stroakmont & Atton, each submitted an appeal with
the Stuttgart Court (the "Appeals") against the Judgment.
On June 23, 2023, Adjupharm filed its statement of grounds for appeal with the Higher Regional Court of Stuttgart. Adjupharm appeals against the fact
that the directors of Stroakmont & Atton were not sentenced to pay jointly and severally together with Stroakmont & Atton as a result of fraud. Since they concluded the purchase agreement with Adjupharm in the name of Stroakmont & Atton
and there is indication that they did not intend to pay the purchase price from the very beginning, this could be fraudulent inducement, for which they would be personally liable.
Stroakmont & Atton appeals against the judgement and request to reject the payment claim. Furthermore, they appeal against the prohibition of the
set-off. Stroakmont & Atton are of the opinion that there is no such prohibition, and they want to include their alleged counterclaims in the proceedings and to receive a decision for their counterclaim by the court in Stuttgart.
So far, there have not been any instructions from the Court of Appeal and but the Court has scheduled a first oral hearing on
November 14, 2024. The above mentioned court hearing was canceled due to illness of a judge. The court has not yet scheduled a new hearing.
At this stage, the Company management cannot assess its ability to collect the payment awarded in the Judgment and the chances of the claim advancing
or the potential outcome of the Appeal.
UNICLARO GMBH VS. ADJUPHARM
On December 22, 2022, Uniclaro GmbH filed a statement of claim against Adjupharm with the district court in Hamburg. According to the statement of
claim, Uniclaro GmbH is (“Uniclaro”) claiming the payment of the amount of EUR 1,046,010 (including VAT) in exchange for 300,000 Covid-19 rapid.
Uniclaro alleges in this lawsuit that Adjupharm purchased 4.3 million Covid-19 rapid tests of the brand "Clongene" from Uniclaro. Furthermore, Uniclaro
claims that the order was placed verbally on 23.03.2021 and that Adjupharm has already paid for a portion of these tests and received them, but not yet the entire 4.3 million tests. They reserve the right to extend the lawsuit for a further amount
(which they did not specify).
According to Uniclaro's statement of claim the lawsuit does not concern the same purchase price and the same Covid-19 rapid tests as in the Stroakmont
& Atton Claim mentioned above. On 23 February 2023, the Company provided its statement of defense to the court. The statement of defense contains similar arguments to reject the allegations in this respect as in the court proceedings in
Stuttgart about the counterclaims. Adjupharm rejected the claim stating that it did not purchase such an amount if Covid-19 rapid tests, but only small portions on a case-by-case-basis and according to the available cash flow.
35
Management’s Discussion and Analysis
On February 14, 2024, a court hearing took place before the district court of Hamburg, at which the court also took evidence. The
court first heard the managing directors of Uniclaro and Adjupharm. They commented on the events of March 23, 2021, and the alleged purchase. The statements of all managing directors differed from each other. Afterwards, the witness Francesco
Bisceglia, who holds the position of Sales Director at Adjupharm, was also heard. His statement also partially deviated from the statements of all managing directors, but overall, the witness basically testified that the company did not purchase
4.3 million Clungene Tests in the meeting of 23 March 2021.
On April 24, 2024, the Regional Court of Hamburg announced its decision. The judgment is as follows:
| 1. |
Adjupharm was not sentenced. Uniclaro's lawsuit for payment of EUR 1,046,010.00 in exchange for delivery of 300,000 Clungene tests was dismissed.
|
| 2. |
Uniclaro is sentenced to pay Adjupharm EUR 53,990.00 plus interest at 5 percentage points above the German basis rate since 17.01.2023.
|
| 3. |
Uniclaro shall bear the procedural costs.
|
The judgment is not yet final (rechtskräftig). Uniclaro has appealed against the judgment and applied for the
judgment to be overturned and to sentence Adjupharm in accordance with Uncilaro's original application to pay the amount of EUR 1,046,010 (including VAT) in exchange for 300,000 Covid-19 rapid. Furthermore, Uniclaro has requested in its appeal to
dismiss Adjupharm's counterclaim.
Uniclaro essentially argues that the facts stated by the Hamburg Regional Court in its judgment are incorrect and incomplete. As before, Uniclaro is of
the opinion that - allegedly undisputed - an oral agreement for the purchase of Clongene rapid tests was reached on 23 March 2021, but only the number of tests is in dispute. While Adjupharm claims to have only ordered 200,000 tests, Uniclaro
claims that the number of Covid rapid tests ordered was 4.3 million resp. 8.6 million tests. Uniclaro furthermore claims that a written contract was not constitutive and not required for the 4.3 million orders, as there was also no written contract
regarding 200,000 Covid rapid tests and that these were part of the 4.3 million order.
Furthermore, Uniclaro claims that circumstantial evidence (Indizien) in favor of Uniclaro was not taken into account in the evaluation of the evidence
and that the Hamburg Regional Court did not provide adequate reasoning in parts of the judgment, as the court referred to statements by the managing director of Adjupharm and the witness Francesco Bisceglia without giving reasons for this, although
there had been contrary statements by Uniclaro. In addition, Uniclaro claims that witnesses named by Uniclaro were not heard.
With respect to Adjupharm's counterclaim, Uniclaro alleges that the court ignored the burden of proof and failed to consider that Adjupharm bears the
burden of proof and allegedly did not prove its claim.
Adjupharm responded on September 20, 2024, to the grounds of appeal and has requested to dismiss Uniclaro's appeal.
In its response, Adjupharm not only demonstrated the contradictions in Uniclaro's arguments, but also argued that the allegedly
unheard witnesses could not have witnessed the conclusion of the contract because they were not present at the decisive meeting. For this reason, the court did not have to hear these witnesses.
Furthermore, Adjupharm has stated why the court’s judgement is correct and complete since all aspects of the case and in
particular the entire arguments of Uniclaro were evaluated by the court and taken correctly into account in its decision making. Nevertheless, Uniclaro was not able to prove the alleged conclusion of the contract.
So far, there have not been any instructions from the Court of Appeal. The court has not yet scheduled an oral hearing.
At this stage, the Company management cannot assess the chances of the potential outcome of this these proceedings.
36
Management’s Discussion and Analysis
PROCEEDINGS UNDER CCAA
See the Company’s Annual Report, available on the Company’s profile on SEDAR+ at www.sedarplus.ca and on EDGAR at www.sec.gov/edgarfor
a summary of the CCAA Proceedings.
Court materials filed in connection with Trichome's CCAA Proceedings can be found at https://www.ksvadvisory.com/insolvency-cases/case/trichome.
THE REGIONAL LABOR COURT - TEL AVIV (BAT YAM) 17419-04-23
On May 10, 2023, IMC Holdings received a notice that a former employee has recently filed a claim with The Regional Labor Court - Tel Aviv (Bat Yam)
(the "Court") against 3 companies, including IMC Holdings.
On April 4, 2024, IMC Holdings filed its Statement of defense.
A preliminary hearing held on May 6, 2024, before the esteemed Honorable Judge Karin Liber-Levin at The Court. Following the preliminary hearing an
adjusted claim was filed with the Court.
The nature and details of the claim and the adjusted claim are still in the preliminary stages, and IMC Holdings is actively working to comprehend the
full scope of the allegations. At this stage, the Company management cannot accurately assess the potential outcome of the claims or the likelihood of the claims progressing further.
ONTARIO SUPERIOR COURT OF JUSTICE IN CANADA – STATEMENT OF CLAIM
On November 17, 2023, the Company received a copy of a Statement of Claim that was filed in the Ontario Superior Court of Justice in Canada by 35 Oak
Holdings Ltd., MW Investments Ltd., 35 Oak Street Developments Ltd., Michael Wiener, Kevin Weiner, William Weiner, Lily Ann Goldstein-Weiner, in their capacity as trustees of the Weiner Family Foundation (collectively the "MYM Shareholder Plaintiffs") against the Company and its board of directors, Board and officers, (collectively, the "MYM Defendants").
MYM Shareholder Plaintiffs claims that the MYM Defendants made misrepresentations in its disclosures prior to the Company's transaction with MYM in
2021. The MYM Shareholder Plaintiffs are claiming damages that amount to approximately $15,000 and aggravated, exemplary and punitive damages in the amount of $1,000.
The Company has reviewed the complaint and believes that the allegations are without merit.
The Company, together with some of the Defendants brought, on February 22, 2024, a preliminary motion to strike out several significant parts of the
claim (the "Motion") The Motion has not been scheduled by the court.
At this time, the Company's management is of the view that the Motion has merit and is likely to succeed in at least narrowing the scope of the claim
against the Company, and that it may also result in certain of the claims against individuals being dismissed altogether, and if not dismissed narrowed in scope and complexity.
On June 17, 2024, an Amended Statement of Claim was filed in the Ontario Superior Court of Justice in Canada by the MYM Shareholder Plaintiffs. The
Plaintiffs have requested that the Defendants serve a Statement of Defence by November 18, 2024.
37
Management’s Discussion and Analysis
The Company together with the Defendants Oren Shuster, Marc Lustig, Brian Schinderle, and Shai Shemesh served a Demand for Particulars on October 28,
2024, requesting the details of the allegations against the Defendants. The Company is currently awaiting a response from the Plaintiffs.
Given the preliminary stage of the action, and that the Company have not yet received full particulars of the allegations or conducted a full
investigation of the factual defences, it is too early to opine on the merits of the claim or whether it is more likely than not to result in an outflow of funds to the Company and if so, how much. The Company plans to vigorously defend itself
against the allegations. At this stage, the Company management cannot assess the chances of the claim advancing or the potential outcome of this these proceedings.
OFF-BALANCE SHEET ARRANGEMENTS
IM Cannabis had no off-balance sheet arrangements as of September 30, 2024.
TRANSACTIONS WITH RELATED PARTIES
Transactions with related parties mainly includes enterprises owned by directors or major shareholders and enterprises that have a member of key
management in common with us. All the transactions have been reviewed and approved by the Board or another independent committee of the board.
| • |
On April 2, 2019, IMC Holdings and Focus entered into an option agreement (the “Focus Agreement”) pursuant to which IMC Holdings acquired an option to purchase, at its sole discretion and in
compliance with Israeli cannabis regulation, all the ordinary shares held by Messrs. Shuster and Gabay held in Focus at a price equal to NIS 765.67 per ordinary share until April 2029. On November 30, 2023, IMC Holdings sent a request
letter to approve IMC Holding’s exercise of the option and on February 25, 2024, IMCA's approval was obtained. Effective February 27, 2024, IMC Holdings acquired 74% of the ordinary shares of Focus.
|
| • |
The Company is a party to Indemnification Agreement with certain directors and officers of the Company and Trichome to cover certain tax liabilities, interest and penalties arising from the Trichome Transaction. See “Risk Factors - Tax Remittance” section of the MD&A.
|
| • |
On April 17, 2024, R.A. Yarok Pharm entered into a loan agreement with a non-financial institute in the amount of NIS 3,000,000 (approximately $1,082,000). Such a loan bears interest at an annual rate of 15% and mature 12 months from the
date of issuance (the "Loan"). The Loan is secured by the following collaterals and guarantees: (a) a first-ranking floating charge over the assets of A.R. Yarok Pharm, (b) a first-ranking fixed
charge over the holdings (23.3%) of its subsidiary, IMC Holdings, of Xinteza, (c) a personal guarantee by Mr. Oren Shuster, the Company’s Chief Executive Officer and (D) a guarantee by the Company.
|
| • |
On October 12, 2023, Oren Shuster, the CEO (the "Insider") loaned an amount of NIS 500 thousand (approximately $170) to IMC Holdings. The participation of the CEO constituted a “related party
transaction”, as such term is defined in MI 61-101 and would require the Company to receive minority shareholder approval for and obtain a formal valuation for the subject matter of, the transaction in accordance with MI 61-101, prior to
the completion of such transaction. However, in completing the loan, the Company has relied on exemptions from the formal valuation and minority shareholder approval requirements of MI 61-101, in each case on the basis that the fair market
value of the CEO’s loan did not exceed 25% of the market capitalization of the Company, as determined in accordance with MI 61-101. On May 29, 2024, the Company closed a non-brokered private placement (the “Offering”)
of secured convertible debentures of the Company (each, a “Debenture”) for aggregate proceeds of $2,091,977. The Insider has subscribed for an aggregate of $237,214 of Debentures in the Offering. The
Insider’s participation in the Offering (the “Insider Transaction”) is a “related party transaction” as described above.
|
38
Management’s Discussion and Analysis
| • |
On November 12, 2024, the Company announced that further to its press release dated October 4, 2024 (the “October 4 Release”), the Company has closed its previously announced non-brokered private placement offering (the “Offering”) effective November 12, 2024 (the
“Closing Date”) through the issuance of 742,517 Units for gross proceed of C$2,138,448.96 Capitalized terms not otherwise defined
herein have the meanings attributed to them in the October 4 Release.
|
Each Unit was sold at a price of C$2.88 per Unit, calculated based on the deemed price per Share equal to the 10-day volume weighted
average price of the Shares on the Exchange ending on the trading day preceding October 3, 2024, and consisted of one Share and one Warrant.
Each Warrant entitles the holder thereof to acquire one Warrant Share at a price of C$4.32 per Warrant Share, calculated as a 50%
premium to the Offering Price, at any time prior to 5:00 pm (Toronto Time) on the date that is twenty-four months following the Closing Date.
All securities issued under the Offering are subject to: (i) a four month and one day hold period from the date of issuance and (ii)
applicable legends as required pursuant to the United States Securities Act of 1933, as amended.
The Company intends to use the proceeds from the Offering for the repayment of a loan to A.D.I. CAR ALARMS & STEREO SYSTEMS Ltd.
provided to the Company’s subsidiary IMC Holdings Ltd. on October 11, 2022.
The Company also announced that the Company has completed a debt settlement (the “Debt Settlement”) in the amount of US$560,000 approximately C&758,240.00, based on an exchange rate of US$1.00 = C$1.354 as at October 3, 2024, as published
on the website of the Bank of Canada with Oren Shuster, the Company’s Chief Executive Officer, in connection with the Benefit, to preserve the Company’s cash for working capital through the issuance of 110,576 Shares and 152,701 Pre-Funded
Warrants at a deemed price of C$2.88.
Each Pre-Funded Warrant will entitle the holder to purchase one Settlement Share for a price of $0.00001, upon receipt of
shareholder approval to allow Mr. Shuster to become a control person (as defined in the policies of the Exchange).
All securities issued in consideration for the Benefit are subject to: (i) a four month and one day hold period from the date of
issuance and (ii) applicable legends as required pursuant to the United States Securities Act of 1933, as amended.
Oren Shuster, a director and officer of the Company, Shmulik Arbel, a director of the Company and Rafael Gabay, an insider
of the Company, (together, the “Participating Insiders”) each participated in the Offering and Mr. Shuster participated in the Debt
Settlement. Mr. Shuster acquired 194,110 Units, 110,576 Settlement Shares and 152,701 Pre-Funded Warrants, Mr. Arbel acquired 48,349 Units and Mr. Gabay 194,088 Units.
39
Management’s Discussion and Analysis
The participation of the Participating Insiders in the Offering constitutes a “related party transaction”, as such term is defined
in MI 61-101 and would require the Company to receive minority shareholder approval for and obtain a formal valuation for the subject matter of, the transaction in accordance with MI 61-101, prior to the completion of such transaction. However, in
completing the Offering, the Company has relied on exemptions from the formal valuation and minority shareholder approval requirements of MI 61-101, on the basis of subsections 5.5(g) and 5.7(g) – Financial Hardship of MI 61-101, as the Company is
(i) in a situation of serious financial difficulty; (ii) the Transactions are designed to improve the financial position of the Company as (x) the Company would be unable to repay back the loan provided to A.D.I. CAR ALARMS & STEREO SYSTEMS
Ltd. provided to the Company’s subsidiary IMC Holdings Ltd. without the completion of the Offering and (y) would have been unable to obtain Loans without Mr. Shuster personal guaranteeing them; (iii) the circumstances described in Section 5.5(f) of
MI 61-101 are not applicable, and (iv) the Board and independent directors (as such term is defined in MI 61-101) have, acting in good faith, determined that (i) and (ii) apply and the terms of the Transactions are reasonable in the circumstances
of the Company.
The Transactions were approved by the members of the Board who are independent for the purposes of the Transactions, respectively.
No special committee was established in connection with the Transactions; however, the independent members of the Board commissioned a third-party valuator to determine the Benefit.
| • |
Further details will be included in a material change report to be filed by the Company. The Company did not file a material change report more than 21 days before the closing date of the Transactions as the participation of
Participating Insiders in the Offering was not definitively known to the Corporation until closing. In the Company’s view, the shorter period was necessary to permit the Company to close the Transactions in a timeframe consistent with usual
market practice for transactions of this nature and was reasonable and necessary to improve the Company’s financial position. Effective October 4, 2024, the Company has cancelled an aggregate of 31,305 options (“Options”) to purchase Shares, which were previously granted to Board members, officers, employees, advisors and consultants of the Company (each a “Participant”). Management reviewed
the Company’s outstanding Options and determined that certain Options granted to such Participants, at exercise prices ranging from $6.60 to $600 per Share, no longer represented a realistic incentive to motivate such Participants. The
Company has also approved the grant of 31,305 Options to certain eligible persons of the Company, at an exercise price of greater of: (i) the Warrant Exercise Price; and (ii) C$3.00m per Share, with an expiry date of two years from the date
of issuance (the “Option Grants”). The Options Grants vest as follows: one third vest immediately, one third vests on the six-month anniversary and the final one third vests on the twelve-month
anniversary. All securities issued under the Option Grants are subject to a statutory hold period of four months plus one day from the date of issuance, in accordance with the polices of the Exchange.
|
| • |
Effective October 4, 2024, the Company has cancelled an aggregate of 142,784 Share purchase warrants (the “Subject Warrants”) to purchase Shares, which were previously granted to Mr. Shuster.
Management reviewed the Company’s outstanding warrants and determined that the Subject Warrants at an exercise price of US$9.00 per Share, no longer represented a realistic incentive to motivate Mr. Shuster.
|
Other than the aforesaid transactions noted above, the Company had no other transactions with related parties outside of the Group except those
pertaining to transactions with key management personnel and shareholders in the ordinary course of their employment or directorship.
40
Management’s Discussion and Analysis
PROPOSED TRANSACTIONS
There are no proposed transactions as at the date of this MD&A that have not been disclosed.
CRITICAL ACCOUNTING POLICIES
The consolidated financial statements of the Group have been prepared in accordance with International Financial Reporting Standards ("IFRS"), as
issued by the International Accounting Standards Board ("IASB"). The Group's financial statements have been prepared on a cost basis, except for:
- Financial instruments which are presented at fair value
through profit or loss.
- Biological assets which are presented at fair value less
cost to sell up to the point of harvest.
In the process of applying the significant accounting policies, the Group has made the following judgments which have the most significant effect on
the amounts recognized in the financial statements:
Functional currency, presentation currency and foreign currency
The functional currency of the Company is the Canadian dollar ("CAD"). The Group determines the functional currency of each Group entity.
Assets, including fair value adjustments upon acquisition, and liabilities of an investee which is a foreign operation, and of each Group entity for
which the functional currency is not the presentation currency are translated at the closing rate at each reporting date. Profit or loss items are translated at average exchange rates for all periods presented. The resulting translation differences
are recognized in other comprehensive income (loss). Upon the full or partial disposal of a foreign operation resulting in loss of control in the foreign operation, the cumulative gain (loss) from the foreign operation which had been recognized in
other comprehensive income is transferred to profit or loss. Upon the partial disposal of a foreign operation which results in the retention of control in the subsidiary, the relative portion of the amount recognized in other comprehensive income
is reattributed to non-controlling interests.
Transactions denominated in foreign currency are recorded upon initial recognition at the exchange rate at the date of the transaction. After initial
recognition, monetary assets and liabilities denominated in foreign currency are translated at each reporting date into the functional currency at the exchange rate at that date. Exchange rate differences, other than those capitalized to qualifying
assets or accounted for as hedging transactions in equity, are recognized in profit or loss. Non-monetary assets and liabilities denominated in foreign currency and measured at cost are translated at the exchange rate at the date of the
transaction. Non-monetary assets and liabilities denominated in foreign currency and measured at fair value are translated into the functional currency using the exchange rate prevailing at the date when the fair value was determined.
41
Management’s Discussion and Analysis
JUDGMENTS
Determining the fair value of share-based payment transactions
The fair value of share-based payment transactions is determined upon initial recognition by an acceptable option pricing model. The inputs to the
model include share price, exercise price and assumptions regarding expected volatility, expected life of share option and expected dividend yield.
Variable lease payments that depend on an index:
On the commencement date, the Group uses the index rate prevailing on the commencement date to calculate the future lease payments. For leases in which
the Group is the lessee, the aggregate changes in future lease payments resulting from a change in the index are discounted (without a change in the discount rate applicable to the lease liability) and recorded as an adjustment of the lease
liability and the right-of-use asset, only when there is a change in the cash flows resulting from the change in the index (that is, when the adjustment to the lease payments takes effect).
ESTIMATES AND ASSUMPTIONS
The preparation of the financial statements requires management to make estimates and assumptions that influence the application of the accounting
policies and on the reported amounts of assets, liabilities, revenues, and expenses. Changes in accounting estimates are reported in the period of the change in estimate.
The key assumptions made in the financial statements concerning uncertainties at the reporting date and the critical estimates computed by the Group
that may result in a material adjustment to the carrying amounts of assets and liabilities within the next financial year are discussed below.
ASSESSMENT OF GOING CONCERN
The use of the going concern basis of preparation of the financial statements. At each reporting period, management assesses the basis of preparation
of the financial statements. The going concern basis of presentation assumes that the Company will continue its operations for the foreseeable future and be able to realize its assets and discharge its liabilities and commitments in the normal
course of business.
The Group’s current operating budget includes various assumptions concerning the level and timing of cash receipts from sales and cash outlays for
operating expenses and capital expenditures. Restructuring Plans and actions taken in 2023 and in 2024. The Company’s board of directors approved a cost saving plan, to allow the Company to continue its operations and meet its cash obligations. The
cost saving plan consists of cost reduction due to efficiencies and synergies, which include mainly the following steps: discontinued operations of loss-making activities, reduction in payroll and headcount, reduction in compensation paid to key
management personnel (including layoffs of key executives), operational efficiencies and reduced capital expenditures. In 2024, the company is working for fund and/or debt raising and will continue with cost savings effort as well as increased
efficiency.
These conditions raise substantial doubt about the Company’s ability to continue as a going concern. The consolidated financial statements do not
include any adjustments relating to the recoverability and classification of assets or liabilities that might be necessary should the Company be unable to continue as a going concern.
42
Management’s Discussion and Analysis
BIOLOGICAL ASSETS
The Group’s biological assets consist of cannabis plants. The Group capitalizes the direct and indirect costs incurred related to the biological
transformation of the biological assets between the point of initial recognition and the point of harvest. The direct and indirect costs of biological assets are determined using an approach similar to the capitalization criteria outlined in IAS 2,
Inventory. These costs include the direct cost of planting and growing materials as well as other indirect costs such as utilities and supplies used in the cultivation process. Indirect labor for individuals involved in the cultivation and quality
control process is also included, as well as depreciation on growing equipment and overhead costs such as rent to the extent it is associated with the growing space. All direct and indirect costs of biological assets are capitalized as they are
incurred, and they are all subsequently recorded within the line-item cost of revenues on the Group’s statements of profit or loss and other comprehensive income in the period that the related product is sold. The Group then measures the biological
assets at fair value less cost to sell up to the point of harvest, which becomes the basis for the cost of inventory after harvest. The fair value is determined using a model which estimates the expected harvest yield in grams for plants currently
being cultivated, and then adjusts that amount for the expected selling price per gram and for any additional costs to be incurred (e.g., post-harvest costs). The net unrealized gains or losses arising from changes in fair value less cost to sell
during the period are included in the gross profit for the related period and are recorded in a separate line on the face of the Group’s statements of profit or loss and other comprehensive income. Determination of the fair values of the biological
assets requires the Group to make assumptions about how market participants assign fair values to these assets. These assumptions primarily relate to the level of effort required to bring the cannabis up to the point of harvest, costs to convert
the harvested cannabis to finished goods, sales price, risk of loss, expected future yields from the cannabis plants and estimating values during the growth cycle. The Group accretes fair value on a straight-line basis according to stage of growth
(e.g., a cannabis plant that is 50% through its growing cycle would be ascribed approximately 50% of its harvest date expected fair value, subject to wastage adjustments). The fair value of biological assets is categorized within Level 3 of the
fair value hierarchy. For the inputs and assumptions used in determining the fair value of biological assets. The Group’s estimates are, by their nature, subject to change and differences from the anticipated yield will be reflected in the gain or
loss on biological assets in future periods. As of September 30, 2024, and 2023, the Company does not hold biological assets.
BUSINESS COMBINATIONS AND GOODWILL
Business combinations are accounted for by applying the acquisition method. The cost of the acquisition is measured at the fair value of the
consideration transferred on the acquisition date with the addition of non-controlling interests in the acquiree. In each business combination, the Company chooses whether to measure the non-controlling interests in the acquiree based on their fair
value on the acquisition date or at their proportionate share in the fair value of the acquiree's net identifiable assets. Direct acquisition costs are carried to the statement of profit or loss as incurred.
In a business combination achieved in stages, equity interests in the acquiree that had been held by the acquirer prior to obtaining control are
measured at the acquisition date fair value while recognizing a gain or loss resulting from the revaluation of the prior investment on the date of achieving control. Contingent consideration is recognized at fair value on the acquisition date and
classified as a financial asset or liability in accordance with IFRS 9. Subsequent changes in the fair value of the contingent consideration are recognized in profit or loss. If the contingent consideration is classified as an equity instrument, it
is measured at fair value on the acquisition date without subsequent remeasurement.
43
Management’s Discussion and Analysis
Goodwill is initially measured at cost which represents the excess of the acquisition consideration and the amount of non-controlling interests over
the net identifiable assets acquired and liabilities assumed. If the resulting amount is negative, the acquirer recognizes the resulting gain on the acquisition date.
IMPAIRMENT OF FINANCIAL ASSETS
The Group evaluates at the end of each reporting period the loss allowance for financial debt instruments measured at amortized cost. The Group has
short-term financial assets, principally trade receivables, in respect of which the Group applies a simplified approach and measures the loss allowance in an amount equal to the lifetime expected credit losses. The impairment loss, if any, is
recognized in profit or loss with a corresponding allowance that is offset from the carrying amount of the assets.
IMPAIRMENT OF NON-FINANCIAL ASSETS
The Group evaluates the need to record an impairment of non-financial assets whenever events or changes in circumstances indicate that the carrying
amount is not recoverable. If the carrying amount of non-financial assets exceeds their recoverable amount, the assets are reduced to their recoverable amount. The recoverable amount is the higher of fair value less costs of sale and value in use.
In measuring value in use, the expected future cash flows are discounted using a pre-tax discount rate that reflects the risks specific to the asset. The recoverable amount of an asset that does not generate independent cash flows is determined for
the cash-generating unit to which the asset belongs. Impairment losses are recognized in profit or loss. An impairment loss of an asset, other than goodwill, is reversed only if there have been changes in the estimates used to determine the asset's
recoverable amount since the last impairment loss was recognized. Reversal of an impairment loss, as above, shall not be increased above the lower of the carrying amount that would have been determined (net of depreciation or amortization) had no
impairment loss been recognized for the asset in prior years and its recoverable amount. The reversal of impairment loss of an asset presented at cost is recognized in profit or loss.
The following criteria are applied in assessing impairment of these specific assets:
The Group reviews goodwill for impairment once a year, on December 31, or more frequently if events or changes in circumstances indicate that there is
an impairment.
Goodwill is tested for impairment by assessing the recoverable amount of the cash-generating unit (or group of cash-generating units) to which the
goodwill has been allocated. The Company identified the operations in Israel, Canada, and Europe as three separate cash-generating units.
An impairment loss is recognized if the recoverable amount of the cash-generating unit (or group of cash-generating units) to which goodwill has been
allocated is less than the carrying amount of the cash-generating unit (or group of cash-generating units). Any impairment loss is allocated first to goodwill. Impairment losses recognized for goodwill cannot be reversed in subsequent periods.
44
Management’s Discussion and Analysis
LEGAL CLAIMS
A provision for claims is recognized when the Group has a present legal or constructive obligation because of a past event, it is more likely than not
that an outflow of resources embodying economic benefits will be required by the Group to settle the obligation and a reliable estimate can be made of the amount of the obligation.
PUT OPTION GRANTED TO NON-CONTROLLING INTERESTS
When the Group grants non-controlling interests a put option, the non-controlling interests are classified as a financial liability and are not
accorded their share in the subsidiary's earnings. At each reporting date, the financial liability is measured on the basis of the estimated present value of the consideration to be transferred upon the exercise of the put option / based on the
fair value of the consideration. Changes in the amount of the liability are recorded in profit or loss.
DEFERRED TAX
Deferred taxes are computed in respect of temporary differences between the carrying amounts in the financial statements and the amounts attributed for
tax purposes. Deferred taxes are measured at the tax rate that is expected to apply when the asset is realized or the liability is settled, based on tax laws that have been enacted or substantively enacted by the reporting date.
Deferred tax assets are reviewed at each reporting date and reduced to the extent that it is not probable that they will be utilized. Deductible carry
forward losses and temporary differences for which deferred tax assets had not been recognized are reviewed at each reporting date and a respective deferred tax asset is recognized to the extent that their utilization is probable.
Deferred taxes in respect of investment property that is held with the objective of recovering substantially all of the economic benefits embedded in
the investment property through sale and not through use are measured in accordance with the expected manner of recovery of the base asset, on the basis of sale rather than use.
When the Company owns an investment in a single property company and the manner in which the Company expects to dispose of the investment is by selling
the shares of the property company rather than by selling the property itself, the Company recognizes deferred taxes for both inside temporary differences arising from the difference between the carrying amount of the property and its tax basis,
and for outside temporary differences arising from the difference between the tax basis of the investment and the Company's carrying amount of the net assets of the investment in the consolidated financial statements. Taxes that would apply in the
event of the disposal of investments in investees have not been considered in computing deferred taxes, if the disposal of the investments in investees is not probable in the foreseeable future. Also, deferred taxes that would apply in the event of
distribution of earnings by investees as dividends have not been considered in computing deferred taxes, since the distribution of dividends does not involve an additional tax liability or since it is the Company's policy not to initiate
distribution of dividends from a subsidiary that would trigger an additional tax liability. Taxes on income that relate to distributions of an equity instrument and to transaction costs of an equity transaction are accounted for pursuant to IAS 12.
Deferred taxes are offset if there is a legally enforceable right to offset a current tax asset against a current tax liability and the deferred taxes relate to the same taxpayer and the same taxation authority.
45
Management’s Discussion and Analysis
SHARE-BASED PAYMENTS
The Company uses the Black-Scholes option pricing model in determining the fair value of Options issued to employees. In estimating fair value, the
Company is required to make certain assumptions and estimates such as the expected life of the options, volatility of the Company’s future share price, the risk-free rate, future dividend yields and estimated forfeiture rates at the initial grant
date.
ESTIMATED USEFUL LIVES AND DEPRECIATION/AMORTIZATION OF PROPERTY AND EQUIPMENT, AS WELL AS INTANGIBLE ASSETS
Property, plant and equipment are measured at cost, including directly attributable costs, less accumulated depreciation, accumulated impairment losses
and excluding day-to-day servicing expenses. Cost includes spare parts and auxiliary equipment that are used in connection with plant and equipment. A part of an item of property, plant and equipment with a cost that is significant in relation to
the total cost of the item is depreciated separately using the component method.
Depreciation of property, plant and equipment is dependent upon estimates of useful lives and residual values which are determined through the exercise
of judgement and calculated on a straight-line basis over the useful lives of the assets at annual rates.
LEASES
Judgment is used in determining the value of the Company’s right-of-use assets and lease liabilities. The value determined for the Company’s
right-of-use assets and lease liabilities can be materially different based on the discount rate selected to present value the future lease payments as well as the likelihood of the Company exercising extensions, termination, and/or purchase
options. The discount rate used to present value the future lease payments over the life of the lease is based on the Company’s incremental borrowing rate at inception of the lease. This rate is determined by the Company using judgment.
In determining the value of the Company’s right-of-use assets and lease liabilities, the Company assesses future business plans to determine whether to
include certain extension options noted in the lease agreement.
If there is no interest rate implicit in the lease agreement, the Company uses a discount rate that would be charged to a similar borrower, with
similar risk characteristics, in a mortgage loan to purchase the leased facility. This discount rate is used to present value the future lease payments in determining the right-of-use asset and lease liability values at inception of the leases.
DETERMINING THE FAIR VALUE OF AN UNQUOTED FINANCIAL ASSETS AND LIABILITIES
The fair value of unquoted financial assets in Level 3 of the fair value hierarchy is determined using valuation techniques, generally using future
cash flows discounted at current rates applicable for items with similar terms and risk characteristics. changes in estimated future cash flows and estimated discount rates, after consideration of risks such as liquidity risk, credit risk and
volatility, are liable to affect the fair value of these assets.
REVENUE RECOGNITION
Revenue from contracts with customers is recognized when the control over the goods or services is transferred to the customer. The transaction price
is the amount of the consideration that is expected to be received based on the contract terms, excluding amounts collected on behalf of third parties (such as taxes). In determining the amount of revenue from contracts with customers, the Group
evaluates whether it is a principal or an agent in the arrangement. The Group is a principal when the Group controls the promised goods or services before transferring them to the customer. In these circumstances, the Group recognizes revenue for
the gross amount of the consideration. When the Group is an agent, it recognizes revenue for the net amount of the consideration, after deducting the amount due to the principal.
46
Management’s Discussion and Analysis
REVENUE FROM THE SALE OF GOODS
Revenue from the sale of cannabis products is generally recognized at a point in time when control over the goods have been transferred to the
customer. Payment is typically due prior to or upon delivery and revenue is recognized upon the satisfaction of the performance obligation. The Group satisfies its performance obligation and transfers control upon delivery and acceptance by the
customer.
Variable consideration:
The Group determines the transaction price separately for each contract with a customer. When exercising this judgment, the Group evaluates the effect
of each variable amount in the contract, taking into consideration discounts, penalties, variations, claims, and non-cash consideration. In determining the effect of the variable consideration, the Group normally uses the "most likely amount"
method described in the Standard. Pursuant to this method, the amount of the consideration is determined as the single most likely amount in the range of possible consideration amounts in the contract. According to the Standard, variable
consideration is included in the transaction price only to the extent that it is highly probable that a significant reversal in the amount of revenue recognized will not occur when the uncertainty associated with the variable consideration is
subsequently resolved.
Bill-and-hold arrangements:
Due to strict regulations of security, storage and handling large quantities of cannabis products, the Group's customers may request the Group to
retain physical possession of a sold product until it is delivered to the customer at a future point in time. Revenue from bill-and-hold sales is recognized before the product is physically delivered to the customer when all of the following
criteria are met:
| a. |
The reason for the bill-and-hold arrangement is substantive (for example, the customer has requested the arrangement);
|
| b. |
The product is identified separately as belonging to the customer;
|
| c. |
The product currently is ready for physical delivery to the customer.
|
| d. |
The Group does not have the ability to use the product by selling it or delivering it to another customer.
|
CHANGES IN ACCOUNTING POLICIES INCLUDING INITIAL ADOPTION
The following new accounting standards applied or adopted during the nine months ended September 30, 2024, and had no impact on the
Interim Financial Statements:
| a. |
Amendments to IAS 1,"Non-Current liabilities with Covenants and Classification of Liabilities as current or non-current":
|
In January 2020, the IASB issued amendments to IAS 1, regarding the criteria for classifying liabilities with covenants as current
or non-current.
47
Management’s Discussion and Analysis
In October 2022 the IASB issued an additional amendment accordingly a Company has to disclose of the book value of the liability and
information on the financial benchmarks as well as facts and circumstances at the end of the reporting period that may lead to the conclusion that the entity will have difficulty in complying with the financial covenants. The Amendment is
applicable for annual periods beginning on or after January 1, 2024.
The Company has a loan that is presently convertible into Ordinary shares of the Company. The conversion component is classified in
the financial statements as a financial liability.
| b. |
Amendment to IFRS 16, "LEASES ":
|
In September 2022, the IASB issued an amendment to IFRS 16, " Lease Liability in a Sale and Leaseback" ("IFRS 16"), which provides
accounting treatment in the financial statements of the seller-lessee in sale and leaseback transactions when the lease payments are variable lease payments that do not depend on the index or the exchange rate. As part of the amendment, the
seller-lessee is required to adopt one of two approaches to measuring the liability for the lease at the time of first recognition of such transactions. The chosen approach constitutes an accounting policy that must be applied consistently.
The Amendment is applicable for annual periods beginning on or after January 1, 2024.
The Amendment did not have a material impact on the Company interim consolidated financial statements.
| c. |
Amendments to IAS 7 and IFRS 7, "Statement of Cash Flows", and IFRS 7, "Financial Instruments: Disclosures ":
In May 2023, the IASB published amendments to International Accounting Standard 7, Statement of Cash Flows, and IFRS 7, Financial Instruments: Disclosures
(hereinafter: "the amendments"), to clarify the characteristics of addressing the presentation of liabilities and the associated cash flows arising out of supplier finance arrangements, as well as disclosures required for such
arrangements.
The disclosure requirements in the amendments are intended to assist and enable users of the financial statements to assess the effects of supplier financing
arrangements on the entity's obligations as well as on the entity's cash flow and exposure to liquidity risk. The Amendment is applicable for annual periods beginning on or after January 1, 2024.
According to the transition provisions of the Amendments, the Company is not required to provide disclosures in interim periods during the first year of adoption,
and therefore the above Amendments did not have a material impact on the Company's condensed interim consolidated financial statements. However, the Amendments are expected to affect the disclosures of supplier finance arrangements in the
Company's annual consolidated financial statements.
|
| d. |
IFRS 18, "Presentation and Disclosure in Financial Statements":
|
In April 2024, the International Accounting Standards Board ("the IASB") issued IFRS 18, "Presentation and Disclosure in Financial
Statements" ("IFRS 18") which replaces IAS 1, "Presentation of Financial Statements". IFRS 18 is aimed at improving comparability and transparency of communication in financial statements.
IFRS 18 retains certain existing requirements of IAS 1 and introduces new requirements on presentation within the statement of
profit or loss, including specified totals and subtotals. It also requires disclosure of management-defined performance measures and includes new requirements for aggregation and disaggregation of financial information.
48
Management’s Discussion and Analysis
IFRS 18 does not modify the recognition and measurement provisions of items in the financial statements. However, since items within
the statement of profit or loss must be classified into one of five categories (operating, investing, financing, taxes on income and discontinued operations), it may change the entity's operating profit. Moreover, the publication of IFRS 18
resulted in consequential narrow scope amendments to other accounting standards, including IAS 7, "Statement of Cash Flows", and IAS 34, "Interim Financial Reporting".
IFRS 18 is effective for annual reporting periods beginning on or after January 1, 2027, and is to be applied retrospectively. Early
adoption is permitted but will need to be disclosed.
The Company is evaluating the effects of IFRS 18, including the effects of the consequential amendments to other accounting
standards, on its consolidated financial statements.
FINANCIAL INSTRUMENTS
| A. |
Financial instruments are measured either at fair value or at amortized cost. The table below lists the valuation methods used to determine fair value of each financial instrument.
|
|
Financial Instruments Measured at Fair Value
|
Fair Value Method
|
|
|
Warrants liability1
|
Black & Scholes model (Level 3 category)
|
|
|
Investment in affiliates
|
Market comparable (Level 3 category)
|
|
|
Financial Instruments Measured at
Amortized Cost |
||
|
Cash and cash equivalents, trade receivables and other account receivables
|
Carrying amount (approximates fair value due to short-term nature)
|
|
|
Loans receivable
|
Amortized cost (effective interest method)
|
|
|
Trade payables, other accounts payable and accrued expenses
|
Carrying amount (approximates fair value due to short-term nature)
|
Note:
| 1. |
Finance expense (income) include fair value adjustment of warrants, investments, and derivative assets measured at fair value, for the nine months ended September 30, 2024, and 2023, amounted to $24 and $(4,547), respectively.
|
The warrants fair value for September 30, 2024, was measured using the Black & Scholes model with the following key assumptions:
|
Issue date
|
||||||||||||
|
May 2023
|
February 2023
|
May 2021
|
||||||||||
|
Expected volatility
|
48.43
|
%
|
48.43
|
%
|
48.43
|
%
|
||||||
|
Share price (Canadian Dollar)
|
3.22
|
3.22
|
3.22
|
|||||||||
|
Expected life (in years)
|
1.603
|
1.356
|
1.603
|
|||||||||
|
Risk-free interest rate
|
3.82
|
%
|
3.98
|
%
|
3.82
|
%
|
||||||
|
Expected dividend yield
|
0
|
%
|
0
|
%
|
0
|
%
|
||||||
|
Fair value:
|
||||||||||||
|
Per Warrant (Canadian Dollar)
|
$
|
0.025
|
$
|
0.013
|
$
|
0
|
||||||
|
Total Warrants (Canadian Dollar in thousands)
|
$
|
2
|
$
|
11
|
$
|
0
|
||||||
49
Management’s Discussion and Analysis
| B. |
Debentures are measured in fair value for the issuance date. The Debentures fair value for the issuance date was measured using 16.55% IRR and was summarized to $1,975 of Convertible Debt and $327 of conversion option for Convertible
Debt. Finance (income) expense in respect of the Convertible Debt for the nine months ended September 30, 2024, and 2023, amounted to $523 and $nil, respectively.
|
The Group’s exposure to risk for its use of financial instruments are discussed in the Risk Factors.
PROCEDURES AND INTERNAL CONTROL OVER FINANCIAL REPORTING
In accordance with National Instrument 52-109 – Certification of Disclosure in Issuers’ Annual and Interim Filings
(“NI 52-109”) and Rule 13a-15 under the United States Securities Exchange Act of 1934, as amended (the “Exchange Act”), the establishment and maintenance of the
Company’s disclosure controls and procedures (“DC&P”) and internal control over financial reporting (“ICFR”) is the responsibility of management.
Internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and
the preparation of financial statements for external purposes in accordance with applicable IFRS. Internal control over financial reporting should include those policies and procedures that establish the following:
| ● |
maintenance of records in reasonable detail, that accurately and fairly reflect the transactions and dispositions of assets.
|
| ● |
reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with applicable IFRS.
|
| ● |
receipts and expenditures are only being made in accordance with authorizations of management or the Board; and
|
| ● |
reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of the Company’s assets that could have a material effect on the financial instruments.
|
NI 52-109 requires the CEO and CFO to certify that they are responsible for establishing and maintaining DC&P and ICFR for the Company and have
concluded that as at December 31, 2023, those internal controls have been designed and are effective in providing reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements in accordance with
IFRS.
50
Management’s Discussion and Analysis
The Company maintains a set of DC&P designed to provide reasonable assurance that information required to be publicly disclosed is recorded,
processed, summarized and reported on a timely basis. As required by NI 52-109 and Exchange Act Rule 13a-15(b), an evaluation of the design and operation of our DC&P was completed as of December 31, 2023, under the supervision and with the
participation of management, including our CEO and CFO using the criteria set forth in the Internal Control - Integrated Framework (2013), issued by the Committee of Sponsoring Organizations of the Treadway Commission. Based upon this evaluation,
our CEO and CFO concluded that as at December 31, 2023, the Company’s DC&P and ICFR were effective.
There have been no changes to the Company’s ICFR during the three and nine months ended September 30, 2024 that have materially affected, or are likely
to materially affect, the Company’s ICFR.
LIMITATIONS OF DISCLOSURE CONTROLS AND PROCEDURES AND INTERNAL CONTROL OVER FINANCIAL REPORTING
The Company’s management, including the CEO and CFO, believe that due to inherent limitations, any DC&P or ICFR, no matter how well designed and
operated, can provide only reasonable, not absolute, assurance of achieving the desired control objectives. Further, the design of a control system must reflect the fact that there are resource constraints, and the benefits of controls must be
considered relative to their costs. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that any design will not succeed in achieving its stated goals under all potential future conditions. Accordingly,
because of the inherent limitations in a cost- effective control system, misstatements due to error or fraud may occur and not be detected. Additionally, management is required to use judgment in evaluating controls and procedures.
LIMITATION ON SCOPE OF DESIGN
In accordance with Section 3.3 of National Instrument 52-109 – Certification of Disclosure in Issuers’ Annual and Interim Filings (“NI 52-109”), the Company has limited the design of its DC&P and ICFR to exclude the controls, policies and procedures of Oranim Plus (the “Excluded Entity”), acquired
by the Company or by one of its subsidiaries within 365 days of the end of the period ended December 31, 2023.
As of September 30, 2024, the Company has implemented its DC&P AND ICFR in all its subsidiaries.
RESTRUCTURING
Current Israeli law requires prior approval by the IMCA, a unit of the MOH, of the identity of any shareholder owning 5% or more of an Israeli company
licensed by the IMCA to engage in cannabis-related activities in Israel. For a number of reasons, including the opportunity to leverage a network of multiple Israeli licensed producers cultivating under the IMC brand, and in contemplation of a
“go-public transaction” to geographically diversify the Company’s share ownership, IMC Holdings restructured its organization on April 2, 2019 (the “IMC Restructuring”) resulting in the divestiture to Oren
Shuster and Rafael Gabay of its interest in Focus, which is licensed by the IMCA to engage in cannabis-related activity in Israel.
IMC Holdings retained an option with Messrs. Shuster and Gabay to re-acquire the interest sold in Focus at its sole discretion and in accordance with
Israeli cannabis regulations, within 10 years of the IMC Restructuring date (the “Focus Agreement”). The Focus Agreement sets an aggregate exercise price of NIS 765.67 per share of Focus, totaling NIS
2,756,500, equivalent to the price paid by Messrs. Shuster and Gabay for the acquired interests in Focus at the time of the IMC Restructuring. On November 30, 2023, IMC Holdings exercised its option to purchase the 74% interest in Focus held by
Oren Shuster and Rafael Gabay by submitting a request to IMCA, which approved the transaction on February 25, 2024. IMC Holdings provided all necessary information and intends to timely file all required notices with the tax authorities according
to applicable law. On February 25, 2024, concurrently with the exercise of IMC Holdings' option, Ewave Group Ltd. exercised its option to receive Mr. Tal Tregerman's 26% holding in Focus in lieu of loan repayment, in accordance with the loan
agreement between the parties. IMC Holdings intends to purchase the remaining 26% holding in Focus from Ewave Group Ltd, pending all necessary organizational and regulatory approvals.
51
Management’s Discussion and Analysis
As part of the IMC Restructuring, on April 2, 2019, IMC Holdings and Focus entered into an agreement, as amended on January 1, 2021 (the “IP Agreement”), which provides for Focus’s obligation to use the IMC brand for the sale of any cannabis plant and/or cannabis product produced by Focus, either alone or together with other sub-contractors engaged
by Focus through the IP Agreement. On February 26, 2024 the parties to the IP Agreement executed a cancellation note, thereby cancelling the IP Agreement as of the signing date.
Focus is also obligated through a services agreement, as amended on January 1, 2021, (the “Services Agreement”)
to use IMC Holdings for certain management and consulting services including: (a) business development services; (b) marketing services; (c) strategic advisory services; (d) locating potential collaborations on a worldwide basis; and (e) financial
analysis services through the Services Agreement.
Under the Services Agreement, the Parties apply an arm’s length markup on total costs, on a quarterly basis, in accordance with a transfer pricing
analysis to be updated from time to time, as consideration for the provision of such services.
In addition, Rosen and Pharm Yarok signed a services agreement to use IMC Holdings for certain services such as administrative, financial, legal, and
headquarters services. In consideration for the services Rosen and Pharm Yarok shall pay IMC Holdings on a quarterly basis (unless agreed otherwise by the Parties) an amount equal to an arm’s length calculation as determined from time to time. The
charges for the services provided by IMC Holdings will be allocated based on Key Performance Indicators (KPIs).
ISRAELI MARKET DEVELOPMENT 2013-2024
According to Israeli Ministry of Health, as of October 2024, there are 115,072 medical cannabis licensed patients in Israel. A monthly prescription
of 4,634,000 grams of medical cannabis were recorded in October 2024 a decrease of 540,000 grams of cannabis from October 2023.1
The chart below reflects the growth in licensed medical cannabis patients in Israel between February 2015 to October 2024.2
1 Israel Ministry of Health – licensed patients’
data as of October 7 2024 - chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj/https://www.gov.il/BlobFolder/reports/licenses-status-oct-2024/he/subjects_cannabis_docs_licenses-status-oct-2024.pdf
2 Ministry of Health – licensed patients’ data
as of October 2024 - chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj/https://www.gov.il/BlobFolder/reports/licenses-status-oct-2024/he/subjects_cannabis_docs_licenses-status-oct-2024.pdf
52
Management’s Discussion and Analysis
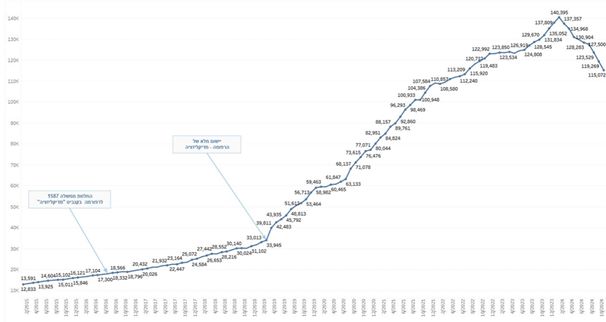
REGULATORY FRAMEWORK IN ISRAEL
In Israel, cannabis is currently defined as a “dangerous drug” according to the Dangerous Drugs Ordinance3 (“DDO”) and the 1961 Single Convention on Narcotic Drugs (“Narcotics Convention”), to
which Israel is a signatory. However, both the DDO and the Narcotics Convention allow for the use of cannabis for medical or research purposes under a supervised and controlled regime. The competent regulatory authority in Israel in all matters
concerning the oversight, control and regulation of cannabis for medical production, consumption, and research in Israel is the IMCA, established by Government Res. No. 3069.4 The production, distribution and consumption of adult-use recreational cannabis products is currently illegal in Israel.
Patient Medical Consumption
The use of cannabis is allowed for patients and for medical purposes, in respect of certain medical conditions, under a special approval of the MOH.
Procedure 1065 of the IMCA sets out a list of medical conditions that are allowed to be treated with medical cannabis products.
Such authorized medical conditions are examined and updated from time to time, and include, among others, cancer, pain, nausea, seizures, muscle spasms, epilepsy, Tourette syndrome, multiple sclerosis, amyotrophic lateral sclerosis, and
post-traumatic stress disorder.
Licensing and Authorization for Commercial Activities in the Medical Cannabis Field
In December 2017, the IMCA issued regulations that standardized the licensing process for any cannabis related activity (the “Road Map”).6 Pursuant to the Road Map, each operation in the medical cannabis field, including the propagation,
cultivation, products manufacturing, storage and distribution to licensed pharmacies, and distribution from licensed pharmacies to licensed patients, requires compliance with the provisions of applicable laws, including the procurement of an
appropriate license under the DDO from the IMCA and the maintenance of such license in good standing. Cannabis licenses may not be transferred, exchanged or assigned without the prior approval of the IMCA. The licenses are valid for a period of up
to 3 years and can be renewed with the approval of the IMCA only.
3 Cannabis is listed in schedule 1 of the Dangerous Drugs Ordinance [New Version], 1973 [ in
English]
https://www.health.gov.il/LegislationLibrary/Samim_01_EN.pdf
4 Israeli Government Res. No. 3609 [in Hebrew], August 7th, 2011 https://www.gov.il/he/departments/policies/2011_des3609
5 Ministry of Health Pharmaceutical Division Policy Number 106 – Licenses for Use of Cannabis
https://www.health.gov.il/hozer/CN_106_2019.pdf (in Hebrew)
53
Management’s Discussion and Analysis
The IMCA has issued a set of directives containing procedures and requirements for applicants for cannabis related activity licenses and has authorized
certain entities to issue official certificates upon compliance with such directives. These directives include (i) Directive 150 (GSP Standard certification); (ii) Directive 151 (GAP Standard certification); (iii) Directive 152 (GMP Standard
certification); and (iv) Directive 153 (GDP Standard certification). Regular and periodic examinations are conducted for licensed entities, in order to ensure compliance with the analytical standards and the level of quality required during each of
the phases of production and distribution of medical cannabis.
Medical Cannabis Imports and Exports
The Narcotics Convention governs the import and export of cannabis between member countries. Since Israel is a member country, any export and import of
cannabis is subject to the Narcotic Convention.
In October 2020, the IMCA issued an updated procedure, titled “Guidelines for Approval of Applications for Importation of
Dangerous Drug of Cannabis Type for Medical Use and for Research” (“Procedure 109”), describing the application requirements for cannabis import licenses for medical and research purposes. Therefore, each
import of medical cannabis is to be approved by the IMCA issuing a specific import permit for each imported shipment, rather than a general license for import. An application for import of medical cannabis can be submitted by an entity licensed by
the IMCA for the conduct of medical cannabis related activity. The Israeli government approved the export of pharmaceutical-grade cannabis and cannabis-based products on January 27, 2019,7 and in December 2020, the IMCA published guidelines for the medical cannabis export permit application process.8.
Legalization of Adult-Use Recreational Cannabis and CBD for Non-Medical Purposes in Israel
Currently, adult-use recreational cannabis use in Israel and CBD for non-medical use is illegal. In November 2020, an Israeli government committee
responsible for advancing the cannabis market reform published a report supporting and recommending the legalization of adult-use recreational cannabis in Israel. The Israeli parliament dissolved since then without applying the committee’s’
recommendations and all legislative initiatives were suspended. However, the new government, formed on June 13, 2021, declared, and settled in the coalition agreement, its commitment to legalization of adult-use recreational cannabis. Since the
formation of the new government, several legislative initiatives were filed, including for the decriminalization of the possession of cannabis for individual recreational adult-use and the legalization of CBD for non-medical use. In February 2022,
a Ministry of Health committee contemplated the legality of CBD and published its recommendation that CBD should be excluded from the DDO. The main recommendations of the committee were adopted by the Minister of Health, however, to date, the
Minister has not enacted an order directing that CBD be removed from the DDO. On April 1, 2022, new regulations came into force which deemed the previously criminal offences of cannabis possession and use for self-consumption into administrative
offences, which do not impact a criminal record, and limited the penalty to a monetary fine only.
6 Directive 107 - Guidelines for the process of licensing the practice of cannabis for medical
use, as amended on October 2020 [
Hebrew] - https://www.health.gov.il/hozer/CN_107_2019.pdf
7 Directive 4490 [Hebrew] - https://www.gov.il/he/departments/policies/dec4490_2019
8 Directive 110, December 2020 [Hebrew] - https://www.health.gov.il/hozer/CN_110.pdf
54
Management’s Discussion and Analysis
Previous Regime and Price Control
Until September 2019, under the previous regime, patients licensed for consumption of medical cannabis products by the IMCA
received all of their medical cannabis products authorized under their respective licenses at a fixed monthly price of NIS 370, regardless of each patient’s authorized amount. Since September 2019, under the new regime, licenses to patients were no
longer entitling them for such fixed monthly price. However, some medical cannabis patient licenses granted under the previous regime remain valid, entitling their holders to receive medical cannabis products pursuant to the price controls and
supplier restrictions of the former regime. All licenses under the previous regime expired in Q1 2022.
Regulatory Reform from Licenses to Prescriptions for Medical Treatment of Cannabis
In August 2022, the MOH published a draft outline of the transition reform from licenses to prescriptions for medical treatment of
cannabis (the “Proposed Outline”). On June 13, 2023, the health committee of the Knesset approved The Dangerous Drugs Regulations (Amendment), 2023 (hereinafter
referred to as the "Regulations Amendment""), which entail a model change from issuing licenses to prescriptions permits following the publication of the Proposed Outline9. The Regulations Amendment allows accessibility and significant bureaucratic relief for patients. The purpose of the new prescription model (as defined below)
is to enable qualified specialist doctors (excluding general practitioner, family physician, internal physician and pediatrician) to write prescriptions for medical cannabis for patients under the supervision of health care providers (widely known
as Kupat Holim), without requiring a usage license from the Ministry of Health (hereinafter referred to as "The New Prescription Model").
The main changes in the Regulations Amendment are: (i) any specialized doctor can issue permits without the need for specialized
training; (ii) the permits for the use of cannabis will be in the form of prescriptions, and not in the form of licenses from the MOH as the current framework requires; (iii) cannabis products can be sold in any pharmacy, and not only in pharmacies
that have received a special permit from the IMCA and a license from the MOH. The Regulations Amendment will come into effect within 180 days of their publication. To the best of the Company's knowledge, the indications approved as part of the
Regulations Amendment encompass various conditions, such as oncological diseases, active inflammatory bowel disease, AIDS, Multiple Sclerosis, Parkinson's disease, Tourette syndrome, epilepsy, autism, and dementia.
On December 8, 2023, the Company announced a 3-month delay of the anticipated medical cannabis reform announced by the Israeli
ministry of health on August 7, 2023 (the "Reform"). Due to the Israel-Hamas war, the anticipated implementation of the medical cannabis regulatory reform, originally scheduled for December 29, 2023, has been
postponed by three months. The new regulations were designed to alleviate many of the stringent restrictions in the sector, thereby enhancing access to medical cannabis for patients.
9 [Hebrew] - https://www.gov.il/he/Departments/policies/reform-of-drug-prescription
55
Management’s Discussion and Analysis
On April 1, 2024, the Company announced the implementation of the medical cannabis regulatory reform in Israel as of April 1,
2024.
The Reform will be implemented in phases, as approved, and announced by the Israeli Ministry of Health. The key aspects of the
initial phase, commencing today, April 2024, are as follows:
1. Change in the prescription process: patients with a wide range of diseases and medical conditions from Oncology to Parkinsons
will no longer be required to obtain a license to receive medical cannabis. Patients will receive a prescription similar to those for other prescription medications. Pain and PTSD are not included in the Reform yet.
2. Medical cannabis will now be prescribed through the HMO's, Israel's public healthcare system: until the Reform, cannabis could
not be prescribed through the HMO's which cover the majority of the Israeli population.
3. The number of prescribing physicians is expected to increase: as of today, HMO physicians, who are dully trained and certified
within their field of expertise, can prescribe medical cannabis as a first line treatment, as opposed to a last resort, based on medical discretion for the approved indications.
4. The cost for prescription is anticipated to be reduced: the Ministry of Health limited the cost for a medical cannabis
prescription.
For the full report published by the Ministry of Health see (in Hebrew)- https://www.health.gov.il/hozer/mmk152_2016.pdf.
“Anti-Dumping” investigation into cannabis imports from Canada
A notice on the Israeli Government’s website dated January 18, 2024, was addressed to 10 different Canadian cannabis producers: Village Farms
International, Organigram Holdings, Tilray Canada, Hexo Corp (owned by Tilray), The Green Organic Dutchman, Canopy Growth Corporation, SNDL Inc., Cronos Group, Auxly Cannabis Group, Decibel Cannabis, and all the medical cannabis manufacturers in
Canada who export their goods to Israel.
56
Management’s Discussion and Analysis
The Commissioner for Trade Levies at the Ministry of Economy and Industry (the "Commissioner"), announced by
virtue of his authority according to Section 24(d) of the Law on Trade Levies and Defence Measures, 5591 – 1991, of his decision to open an investigation on his own initiative into the export of cannabis from Canada, after he found that special
circumstances of actual damage exist or the probability of actual damage to the local manufacturing industry exist. The notice dated January 15, 2024 also included a letter sent to Michael Mancini, the Chief Commercial Counselor with the Embassy of
Canada,

informing of the investigation. The Ministry of Economy and Industry issued a formal notice to the public to respond to questionnaires regarding the
"Anti-Dumping" investigation.
Further to several requests received from the parties involved in the investigation and in accordance with section 27(b) of the Law on Trade Levies and
Defense Measures, 1991 which states that "The Commissioner may, for special reasons that shall be recorded, extend the period specified in subsection (a) by an additional period that shall not exceed 30 days.", the Commissioner decided that special
conditions exist for extending the deadline for the submission of the required materials as part of the investigation for 10 days until March 10, 2024, due to constraints presented by the parties following the "Iron Swords" war. The main reasons
for the delays in the preparation of the materials were due to the absence of many workers as part of the extensive recruitment in Israel for the reserve service and due to the unique complexity of the Israeli cannabis market where many players are
required to submit data, both as producers and importer. The Company has submitted the relevant questionnaires regarding its subsidiaries Focus and IMC Pharma, which are included in the investigation, as well as for its subsidiary Rosen Highway
which is not included but is a significant importer in Israel.
On June 18, 2024, the Ministry of Economy and Industry announced that it has decided to postpone the final deadline for obtaining its preliminary
decision until July 18, 2024.
On July 10th, 2024, the Commissioner published a preliminary decision
regarding the investigation and findings determining that there is dumping and consequent injury, on the basis of best information available. The Company is evaluating the preliminary decision and its potential impact on the Company and its
subsidiaries. Focus And IMC Pharma submitted their response on August 23, 2024, as required by the preliminary decision.
As part of the preliminary decision, the Commissioner determined that a temporary guarantee is not necessary at this stage, and the Company is now
awaiting the Commissioner’s final decision. This decision must be approved by the Ministry of Economy’s Director General, following consultation with the Ministry of Finance’s Budgets Director. The local growers have filed an administrative
petition against the Commissioner’s decision not to impose a temporary guarantee. The company submitted a request to the court to join the petition to argue against the claims of the local growers and the request was approved by the court. A
hearing on the petition has not yet been scheduled. The Company will file its arguments to the court on November 21, 2024.
On November 10, 2024, the Commissioner published the final report on the investigation into cannabis imports from Canada, recommending the imposition
of tax levies.
According to the recommendations, a tax of 175% will be imposed on cannabis imports from Canadian companies that did not cooperate with the
investigation, while major importers that participated will be subject to lower tax rates, starting at 2% and increasing incrementally. The Company is currently reviewing these recommendations and considering steps to prevent or mitigate the final
decision.
57
Management’s Discussion and Analysis
REGULATORY FRAMEWORK IN GERMANY
On March 10, 2017, the German federal government enacted bill Bundestag- Drucksache 18/8965 – Law amending narcotics and other regulations that amended
existing narcotics legislation to recognize cannabis as a form of medicine and allow for the importation and domestic cultivation of medical cannabis products. Under the updated legislation, cannabis is listed in Annex 3 to the Federal Narcotics
Act (“BtMG”) as a “marketable narcotic suitable for prescription”. Until the Act on the Handling of Consumer Cannabis (Consumer Cannabis Act - KCanG) came into force on 1 April 2024, legalization in Germany
applied only to cannabis for medicinal purposes under state control in accordance with the Narcotic Convention. Currently, the production, distribution, exportation and importation of medical cannabis products in Germany is legal, subject to
regulations and licensing requirements. Operations involving adult-use recreational cannabis products became legal under certain conditions defined in the KCanG. This development has its origins in the fact that the current German government has
declared in the coalition agreement at the end of 2021 its intention to open up the German market also in the adult-use recreational market. In October 2022, a key points paper10 on the controlled supply of cannabis to adults for consumption purposes, although a restructuring of the existing regulatory framework on cannabis in general was also discussed, published by
the cabinet, which was submitted to the European Union Commission for a preliminary legal examination. In this respect, the Federal Government issued a declaration of interpretation with regard to existing international agreements governing the
adult-use recreational cannabis usage and submitted a draft law to the European Union Commission within the framework of a notification. After a long political debate, the German Bundestag approved the federal government's draft law "on the
controlled use of cannabis" (BT Drs. 20/870411, BT Drs. 20/876312, BT-Drs. 20/1042613) on Friday, 23 February 2024. The
draft law (BT Drs. 20/8704) then came into force on 1 April 2024. An adjustment has already been made by Article 1 of the Act of 20 June 2024 (BGBl. 2024 I No. 207)14. Some components of the KCanG, which deal with so-called consumer cannabis, came into force on 1 July 2024 (such as the possibility to apply for a permission to grow by and distribute recreational
cannabis to members of a cultivation association. The entry into force of the law also had direct consequences for medicinal cannabis, which is the subject matter of Art. 2 (Medical Cannabis Act - MedCanG) and 3 (BtMG) of the law. With the entry
into force, cannabis is no longer a narcotic by definition and is therefore no longer subject to the BtMG. The definition in Annex 3 of the BtMG was be replaced by that in Section 2 MedCanG: "Cannabis for medical
purposes: plants, flowers and other parts of plants belonging to the genus Cannabis that are grown for medical purposes under state control in accordance with Articles 23 and 28(1) of the Single Convention on Narcotic Drugs of 1961 of 30 March
1961 (Federal Law Gazette 1973 II p. 1354), as well as delta-9-tetrahydrocannabinol including dronabinol and preparations of all the aforementioned substances". However, the narcotics regulations were replaced by comparable regulations and
authorisations. The Federal Institute for Drugs and Medical Devices (BfArM) will remain responsible for the latter as a higher federal authority. From a regulatory perspective, medicinal cannabis remains a medicinal product or an active
pharmaceutical ingredient, meaning that the requirements under medicinal product law will remain in place. As a result, the marketing of irradiated products continues to require a marketing authorisation in accordance with the Ordinance on
Medicinal Products Treated with Radioactive or Ionising Radiation (AMRadV). Only the narcotics licence pursuant to Section 3 BtMG is replaced by a new licence pursuant to the Medicinal Cannabis Act (MedCanG) (see Section 1), which, however, largely
corresponds to the previous provisions of the BTMG regarding the application process and general regulations. However, there are the following differences that are new due to the entry into force: Medicinal cannabis no longer has to be stored and
transported like a narcotic. The corresponding safety precautions no longer apply, meaning that compliance with the provisions of pharmaceutical law is sufficient. The so-called semi-annual reports will be replaced by annual reports. The
requirements for the person responsible for medicinal cannabis are slightly reduced compared to those for narcotics. A prescription of medicinal cannabis is possible without the need to use the form for prescription for narcotics. A normal
prescription is sufficient. This is just an overview – we would be pleased to comment on this in more detail separately. However, it is likely to be of great importance that the cultivation of medicinal cannabis based on Section 17 MedCanG is no
longer subject to public tenders, but - like the trading licence - is ultimately subject to a two-stage authorisation (at state level regarding the pharmaceutical regulations and at federal level with regard to the fact that it is medicinal
cannabis).
10 https://www.bundesgesundheitsministerium.de/fileadmin/Dateien/3_Downloads/Gesetze_und_Verordnungen/GuV/C/Kabinettvorlage_Eckpunktepapier_Abgabe_Cannabis.pdf
(in German language).
11 https://dserver.bundestag.de/btd/20/087/2008704.pdf
(in German language).
12
https://dserver.bundestag.de/btd/20/087/2008763.pdf (in German language).
13 https://dserver.bundestag.de/btd/20/104/2010426.pdf
(in German language).
14
https://www.recht.bund.de/bgbl/1/2024/207/VO.html.
58
Management’s Discussion and Analysis
Medical cannabis in Germany must comply with the corresponding monographs of the German and European pharmacopoeia. Currently, there are still
(non-harmonised) national pharmacopoeial monographs for cannabis flowers (e.g. in the German Pharmacopoeia (Deutsches Arzneibuch (DAB)) and cannabis extracts (DAB) in the EU. The Committee on Herbal Medicinal Products (HMPC) as the European
Medicines Agency’s (EMA) committee responsible for compiling and assessing scientific data on herbal substances, preparations and combinations, announced that in view of uniform EU quality requirements (including with respect to import and export
of cannabis), further European Pharmacopoeia (Ph. Eur.) Cannabis monographs are in preparation.
The European Pharmacopoeia (Ph. Eur.) Suppl. 11.5 is published and contains the new Ph. Eur. Monograph on cannabis flowers and the new Ph. Eur.
Monograph on Cannabidiol (CBD). According to the current status, the Ph. Eur. Monograph on Cannabis Flowers shall replace the currently existing national monographs (NL, DK, D and CH) from the official implementation date (1 July 2024). According
to the BfArM, the texts of addendum 11.5 in English and French have been declared provisionally applicable. However, the German translation and the announcement also provide for transitional regulations that will make the use of the monograph in
the DAB legal until mid-2025. The new monograph on cannabis flowers includes Starting materials for the production of extracts, medicinal products that can be prescribed as such (herbal medicinal products) that are taken by patients by inhalation
or oral administration. There are not entirely irrelevant changes compared to the German monograph.
All BtMG permit applications had to specify the strains and estimated quantities of medical cannabis involved and any subsequent changes had to be
reported to the Federal Opium Agency of Germany. The same applies regarding Sections 7, 8 MedCanG in relation to the authorisation to trade in medicinal cannabis, although it is now apparent that no expected annual quantities are to be specified.
However, it can be assumed that the BfArM nevertheless enquire about these due to the (albeit somewhat reduced compared to the BtMG) reporting obligations in Sections 16 and 17 MedCanG and the Foreign Narcotics Trade Regulation, which remains
applicable (see Section 14 MedCanG).
CBD is neither a rael subject to the KCanG nor to the MedCanG. Only in Section 1 No. 3 KCanG is there a definition and in Section 1 No. 8 b) KCanG the
exemption of CBD from the term cannabis and in Section 2 para. 2 No. 1 KCanG the exemption from the prohibition of extraction of the cannabis plant, which permits the extraction of CBD, even if it does not contain any further regulations on CBD in
isolation. With regard to synthetic CBD, a different set of regulations is important: the handling of cannabimimetics/synthetic cannabinoids is prohibited in accordance with Section 2 of the Annex in conjunction with Section 3 of the New
Psychoactive Substances Act (NpSG). Product-specific regulations relating to CBD can be found in other regulations. Thus, Annex 1 of the Ordinance on the Prescription of Medicinal Products stipulates that CBD is in principle subject to prescription
but does not specify a minimum quantity or a specific dosage form. If we look at the food sector, a distinction is made between products that naturally contain CBD and those that consist of or contain extracted CBD; the European Commission
considers the latter to be novel foods under Regulation (EU) 2015/2283, which require authorisation before being placed on the market. Although applications for such authorisation have been submitted, the European Commission believes that they
contain at least insufficient data on safety in food use, meaning that none of the applications can currently lead to authorisation. Against this background, various products containing CBD can be found on the German market. There are currently
various court decisions that problematise CBD in foods (especially food supplements) and in cosmetics (especially mouth oil). On the one hand, CBD is regarded as a medicinal product or as a novel food subject to authorisation and therefore
unsuitable for use in a foodstuff, and on the other hand as unsuitable for cosmetic use in the mouth, as CBD would ultimately be consumed in this case (like a foodstuff and therefore to be regarded as foodstuff).
59
Management’s Discussion and Analysis
Cultivation in Germany and Distribution of Medical Cannabis Cultivated in Germany
The Past:
The Federal Opium Agency of Germany’s Federal Institute for Drugs and Medical Devices (“BfArM”) formed a
cannabis division (the “Cannabis Agency”) to oversee cultivation, harvesting, processing, quality control, storage, packaging and distribution to wholesalers, pharmacists and manufacturers. The Cannabis
Agency also regulated pricing of German-produced medical cannabis products and served as an intermediary of medical cannabis product sales between manufacturers, wholesalers and pharmacies on a non-profit basis so far. In late 2018, the Cannabis
Agency issued a call for tenders to award licenses for local medical cannabis cultivation and distribution of German-cultivated medical cannabis products (the “German Local Tender”). The Cannabis Agency
served as an intermediary in the supply chain between such cultivation and distribution. In April 2019, three licenses for local cultivation were granted. In consequence three companies in Germany received the permission to cultivate on behalf of
the Cannabis Agency of the BfArM.
Current Situation:
With the entry into force of the MedCanG, the granting of licences for domestic cultivation is no longer subject to tendering but governed by §§ 4 et
seq. MedCanG. The previously time-consuming tendering and awarding of contracts for the domestic cultivation of cannabis for medical purposes by the Cannabis Agency and the subsequent purchase and distribution of the domestic harvest yields by the
Cannabis Agency from the economic operators determined during the tendering procedure are no longer necessary in future. Ultimately, only the corresponding licences in accordance with the MedCanG and the AMG are required in compliance with the
respective conditions and the associated regulations.
Import volumes and procedures
The past and present regime permits the importation of cannabis plants and plant parts for medicinal purposes under state control subject to the
requirements under the Narcotic Convention, according to which, Germany must estimate the expected demand of medical cannabis products for medical and research purposes for the following year and report such estimates to the International Narcotics
Control Board.
As a prerequisite to obtaining a German import license, the supplier must grow and harvest in compliance with EU-GACP-Guidelines and manufacture in
compliance with EU-GMP-Guidelines and certifications, or alternatively, it is a pure EU-GACP product, and the EU-GMP manufacturing steps then take place in Germany. With regard to imports from third countries and the associated testing and
assessment of EU GMP compliance, the relevant pharmaceutical regulations remain in force, which also provide for on-site inspections by the EU authorities, provided that no MRA or similar is in force for the specific product type. All medical
cannabis products imported to Germany must derive from plant material cultivated in a country whose regulations comply with the Narcotic Convention and must comply with the relevant monographs described in the German and European pharmacopeias.
60
Management’s Discussion and Analysis
Dispensing Exclusively via Pharmacies
Medical cannabis products imported pursuant to an import license under the MedCanG and AMG permits are sold exclusively to pharmacies for final
dispensing to patients on a prescription basis as ‘magistral preparations’, a term used in Europe to refer to medical products prepared in a pharmacy in accordance with a medical prescription for an individual patient. Magistral preparations
require certain manufacturing steps in the pharmacy. Such manufacturing steps of the pharmacist typically include the testing and dosing of pre-packaged cannabis inflorescences (typically referred to as “flos”), medical cannabis products for oral
administration (dronabinol), medical cannabis products for inhalation upon evaporation, and medical cannabis-infused teas. In addition to magistral preparations, medical cannabis products are also marketable as pre-packaged, licensed drugs (e.g.
Sativex®).
NO U.S. CANNABIS-RELATED ACTIVITIES
The Group does not engage in any U.S. cannabis-related activities as defined in Canadian Securities Administrators Staff Notice 51-352 (Revised) – Issuers with U.S. Marijuana-Related Activities.
The Company has implemented risk management governance processes that are led by the Board, with the active participation of management, and updates
its assessment of its business risks on an annual basis. Notwithstanding, it is possible that the Company may not be able to foresee all the risks that it may have to face. The market in which IM Cannabis currently competes is complex, competitive
and changing rapidly, and its business is subject to risks inherent in a high growth, heavily regulated enterprise, and the Company has identified certain risks pertinent to the Group’s business that may have affected or may affect the Group’s
business, financial conditions, results of operations and cash flows, as further described throughout this MD&A and under “Risk Factors” in the Company’s Annual Report on Form 20-F for the fiscal year ended December 31, 2023 available on the
Company’s profile on SEDAR+ at www.sedarplus.com and on EDGAR at www.sec.gov/edgar (the “Annual Report”). For additional risk factors, readers are directed to the Annual Report. Sometimes new
risks emerge, and management may not be able to predict all of them or be able to predict how they may cause actual results to be different from those contained in forward looking statements. Readers of this MD&A should not rely upon forward
looking statements as a prediction of future results.
CREDIT RISK
The maximum credit exposure as of September 30, 2024, is the carrying amount of cash and cash equivalents, trade receivables and
other current assets. The Group does not have significant credit risk with respect to outstanding trade receivables. All cash and cash equivalents are placed with major Israeli financial institutions.
Loan receivable credit risk is managed by each loan separately according to the Company’s policy, procedures and control relating
to the borrower’s credit risk management. At the end of each period, the individual loan values are assessed based on a credit risk analysis.
The expected credit loss analysis is generally based on management’s understanding of the borrower’s experience/integrity,
financial health, business plans, capacity, products, customers, contracts, competitive advantages/disadvantages, and other pertinent factors when assessing credit risk. This would also include the assessment of the borrower’s forecasts as well as
taking into consideration any security and/or collateral the Company has on the outstanding balance.
61
Management’s Discussion and Analysis
LIQUIDITY RISK
As of September 30, 2024, the Group's financial liabilities with liquidity risk consist of trade payables and other accounts payable which have
contractual maturity dates within one year, bank loans and, checks receivables and lease liabilities. The Group manages its liquidity risk by reviewing its capital requirements on an ongoing basis. Based on the Group's working capital position at
September 30, 2024, management considers liquidity risk to be high.
CURRENCY RATE RISK
As of September 30, 2024, a portion of the Company's financial assets and liabilities are held in Euro, NIS and USD consist of cash
and cash equivalents in the amount of EUR 809 thousand (approximately $1,225), NIS 1,868 thousand (approximately $681), USD 1 thousand (approximately $1), respectively. The Company's objective in managing its foreign currency risk is to minimize
its net exposure to foreign currency cash flows by transacting, to the greatest extent possible, with third parties in NIS. The Company does not currently use foreign exchange contracts to hedge its exposure of its foreign currency cash flows as
management has determined that this risk is not significant at this point of time.
SHARE PRICE RISK
The Group's investments in unlisted shares are sensitive to market price risk arising from uncertainties about future value of these investments. The
Group manages the price risk through diversification and by placing limits on individual and total investment in shares. The Company's Board of directors reviews and approves all decisions related to investments in shares. At the reporting date,
the Group's exposure to investments in unlisted shares measured at fair value was $2,282.
TAX REMITTANCE
The Company is subject to the provisions of the ITA12 and to review by CRA13. The Company files its annual tax compliance based on its interpretation
of the ITA and CRA’s guidance. There is no certainty that the returns and tax position of the Company will be accepted by CRA as filed. Any difference between the Company’s tax filings and CRA’s final assessment could impact the Company’s results
and financial position.
There can be no assurance that income tax laws or the interpretation thereof in any of the jurisdictions in which the Company operates will not be
changed or interpreted or administered in a manner which adversely affects the Company and its shareholders. In addition, there is no assurance that CRA will agree with the manner in which the Company calculates taxes payable or that any of the
other tax agencies will not change their administrative practices to the detriment of the Company or its shareholders.
By Notice of Assessment for Excise Tax dated October 23, 2023 and covering the period January 1, 2020 to December 31, 2020, the Company was assessed
tax on insurance of $198,687.57, arrears interest of $36,248.62 and a failure to file penalty of $7,947.49 (collectively, the “2020 Assessment”).
62
Management’s Discussion and Analysis
By Notice of Assessment for Excise Tax dated October 23, 2023 and covering the period January 1, 2021 to December 31, 2021, the Company was assessed
excise tax on insurance of $72,944.92, arrears interest of $1,533.75 and a failure to file penalty of $499.48 (collectively, the “2021 Assessment”).
If a person files a Notice of Objection (Excise Tax Act), the CRA cannot take collection action on amounts in dispute until 90 days after the Notice of
Decision is sent to that person. However, interest and penalty continue to accrue on any amount owing.
On November 29, 2023, the Company filed Notices of Objection (Excise Tax Act) to the 2020 Assessment and the 2021 Assessment. Therefore, the CRA cannot
take collection action on the amounts noted above until 90 days after Notices of Decision are sent to the Company.
On April 26, 2024, the Company received a letter from the CRA that the Notice of Assessment for Excise Tax that the Company objected to will be voided
and no outstanding balance will be owed with respect to such assessments. Based on the forgoing, this matter has been resolved to the Company's satisfaction and the objections were finalized.
Cybersecurity risks
The Company’s information systems and its third-party service providers and vendors are vulnerable to increasing threat of continually evolving
cybersecurity risks, resulting in data breaches and data losses. These risks arising from events including without limitation malware, computer viruses, employee error, extortion, malfeasance, system errors, and hacking. In order to minimize the
risk of these events from occurring, the Group is performing timely maintenance, upgrade and replacement of networks, equipment, IT systems and software and other protective measures. However, any failure or delay in maintaining, upgrading or
replacing such systems and software could materially increase the risk of cybersecurity incident and data breach or data loss, and the Company may experience operational delays, information system failures, and/or increases in capital expenses.
Ultimately, the Company’s business, financial condition, operating results and reputation may be impacted adversely by such occurrences.
The Company’s risk and exposure to these matters cannot be fully mitigated because of, among other things, the evolving nature of these threats. As a
result, cyber security and the continued development and enhancement of controls, processes and practices designed to protect systems, computers, software, data and networks from attack, damage or unauthorized access is a priority. As cyber threats
continue to evolve, the Company may be required to expend additional resources to modify or enhance protective measures or to investigate and remediate any security vulnerabilities.
CONSOLIDATION OF CERTAIN FINANCIAL RESULTS UNDER IFRS 10 AND MAINTENANCE OF COMMON CONTROL
The Company complies with IFRS 10, which applies a single consolidation model using a definition of “control” that requires an investor (as defined in
IFRS 10) to consolidate an investee (as defined in IFRS 10) where: (i) the investor has power over the investee; (ii) the investor has exposure or rights to variable returns from involvement with the investee; and (iii) the investor can use its
power over the investee to affect the amount of the investor’s returns.
Subsequent to the restructuring of IMC Holdings on April 2, 2019, the Company analyzed the terms of the contractual agreements with Focus in accordance
with IFRS 10 to conclude whether it should continue to consolidate the accounts of Focus in its financial statements.
63
Management’s Discussion and Analysis
Under IFRS 10, consolidation occurs when an investor can exercise control over an investee. Control is achieved through voting rights or other evidence
of power. Where there are no direct holdings, under IFRS 10, an investor (as defined in IFRS 10) should consider other evidence of power and ability to unilaterally direct an investee’s (as defined in IFRS 10) relevant activities. In view of the
contractual agreements and the guidance in IFRS 10, notwithstanding that the Company has no direct or indirect ownership of Focus Medical, it has sufficient rights to unilaterally direct the relevant activities (a concept known as “de facto
control”), mainly due to the following:
| (a) |
the Company receiving economic benefits from Focus (and the terms of the contractual agreements between the Company and Focus cannot be changed without the approval of IMC Holdings);
|
| (b) |
IMC Holdings holds 74% interest in Focus;
|
| (c) |
Messrs. Shuster and Gabay each being a director of Focus (while Mr. Shuster concurrently being a CEO, director and substantial shareholder of the Company and Mr. Gabay concurrently being a substantial shareholder of the Company); and
|
| (d) |
the Company providing management and support activities to Focus through a services agreement.
|
Accordingly, under IFRS 10, the Company has “de facto control” over Focus, and therefore consolidates the financial results of Focus in the Company’s
financial statements.
Any failure of the Company or Messrs. Oren Shuster and Rafael Gabay to maintain “de facto control” over Focus as defined under IFRS 10 could alter the
Company’s consolidation model, potentially resulting in a material adverse effect on the business, results of operations and financial condition of the Company.
On November 30, 2023, IMC Holdings acted to exercise its option to purchase the divested 74% interest in Focus held by Oren Shuster, and Rafael Gabay
by submitting a request to the "IMCA," an agency operated by the Israeli Ministry of Health that will allow the option exercise. On February 25, 2024, IMCA approved the persons who will be acting on behalf on IMC Holding pursuant to the exercise of
the option, allowing to complete the transaction. On February 26, 2024, IMC Holdings has exercised its option and as of that date, IMC holds 74% in Focus. The Company will continue to consolidate the financial results of Focus in the Company’s
financial statements.
POSSIBLE DIRECT INVOLVEMENT IN THE ISRAELI CANNABIS INDUSTRY
According to current Israeli regulatory medical cannabis framework, any engagement in Cannabis Activities requires receiving the applicable license
from the “IMCA”, an agency operated by the Israeli Ministry of Health, which requires, among other things, pre-approvals by the IMCA (the “IMCA Pre-Approval Requirement”) of the directors, officers and
shareholders holding 5% or more of the shares of the license applicant (“Material Holders”), and of all directors, officers and shareholders that become Material Holders following the grant of the applicable
license. Therefore, if the Company will be considered by the IMCA as directly engaged in Cannabis Activities the aforementioned approvals by the IMCA might apply, on future security holdings, as described above.
Furthermore, any failure of the Company or its shareholders to comply with the IMCA Pre-Approval Requirement may impact the Group’s ability to continue
operating in compliance with any licenses to engage in Cannabis Activities or to renew such licenses. Any inability of the Group to maintain licenses for Cannabis Activities in good standing may result in a material adverse effect on the Group’s
business, financial condition, results of operations and prospects.
COMPANY’S ABILITY TO CONTINUE AS A GOING CONCERN
The Group’s current operating budget includes various assumptions concerning the level and timing of cash receipts from sales and cash outlays for
operating expenses and capital expenditures, including cost saving plans. In 2023 The Company’s board of directors approved a cost saving plan, to allow the Company to continue its operations and meet its cash obligations. The cost saving plan is
continuing in 2024 and the company will continue its efforts for efficiency operations.
64
Management’s Discussion and Analysis
Despite the cost savings plan as described above, the projected cash flows for 2024 indicates that it is uncertain that the Group will generate
sufficient funds to continue its operations and meet its obligations as they become due. The Group continues to evaluate additional sources of capital and financing. However, there is no assurance that additional capital and or financing will be
available to the Group, and even if available, whether it will be on terms acceptable to the Group or in amounts required.
These conditions raise substantial doubt about the Company’s ability to continue as a going concern. The consolidated financial statements do not
include any adjustments relating to the recoverability and classification of assets or liabilities that might be necessary should the Company be unable to continue as a going concern.
CONFLICT AND POLITICAL INSTABILITY IN EASTERN EUROPE
The first part of 2024 has seen significantly higher levels of volatility in global markets due to market participants’ reactions to, and uncertainty
surrounding, the magnitude and timing of government and central bank action to be taken in response to heightened inflation, as well as Russia’s invasion of Ukraine. This volatility has resulted in a decline in the level of activity in the
financial markets. Continued market volatility or uncertainty related to actions taken or to be taken by central banks, a decline in the global macroeconomic outlook, including as a result of Russia’s invasion of Ukraine and the threat, or outbreak
of more widespread armed conflict in Eastern Europe would cause financial market activity to continue to decrease, which would negatively affect the Group’s revenues and capital markets activity.
CONFLICT AND POLITICAL INSTABILITY IN ISRAEL - THE ISRAEL-HAMAS WAR
The Group is vulnerable to the political, economic, legal, regulatory, and military conditions affecting Israel and the Middle East. Armed conflicts
between Israel and its neighbouring countries and territories occur periodically in the region and may adversely affect the Group’s business, results of operations and financial condition. In addition, the Group may be adversely affected by other
events or factors affecting Israel such as the interruption or curtailment of trade between Israel and its trading partners, or any restrictions or pressure on the Group’s partners or customers or others to prevent or discourage them from doing
business activities with Israel or Israeli businesses, a significant downturn in the economic or financial condition of Israel, a significant downgrading of Israel’s internal credit rating, labour disputes and political instability, including
riots, uprisings and government failures. Restrictive laws or policies directed towards Israel or Israeli businesses could have a material adverse effect on the Group’s business, results of operations, financial condition and prospects.
Any armed conflicts, terrorist activities or political instability in the region could adversely affect business conditions, could harm the Group’s
results of operations, and could make it more difficult for us to raise capital. Parties with whom the Group does business may decline to travel to Israel during periods of heightened unrest or tension, forcing the Group to make alternative
arrangements when necessary in order to meet our business partners face to face. In addition, the political and security situation in Israel may result in parties with whom we have agreements involving performance in Israel claiming that they are
not obligated to perform their commitments under those agreements pursuant to force majeure provisions in such agreements. Further, in the past, the State of Israel and Israeli companies have been subjected to economic boycotts. Several countries
still restrict business with the State of Israel and with Israeli companies. These restrictive laws and policies may have an adverse impact on our operating results, financial condition or the expansion of our business.
65
Management’s Discussion and Analysis
Furthermore, under Israeli law, citizens and permanent residents of Israel are obligated to perform military reserve duty for extended periods of time
and are subject to being called to active duty at any time under emergency circumstances. In response to increased hostilities, there have been periods of significant call-ups of military reservists.
On October 7, 2023, a war between the terror organization Hamas and Israel began. This war has an impact on the Company's business operations. The
Company has also suffered a negative impact continuing also in Q2 2024. There might be a potential positive effect in the medium to long term. The Company has experienced damages to its ability to function, affecting various aspects, including
employees, supplies, imports, sales, and more.
While there are damages, it is still too early to fully assess the extent of their impact. It is possible that there will be additional call-ups in the
future, which may include officers and key personnel of the Group’s, which could disrupt business operations for a significant period of time.
JUDICIAL AND LEGISLATIVE REFORMS IN ISRAEL
Israel is undergoing political and social instability relating to the judicial and legislative reforms proposed by the current government, creating
certain instability and uncertainty. This instability which has a certain effect on the activity of the financial markets may cause material impact on the Groups’ ability to operate in the Israeli market, which derives, among other, from: exposure
to currency exchange rate and interest rate, reduced sales due to disruptive days and lower probability for capital investments.
on August 7, 2023, the Israeli ministry of health announced the anticipated medical cannabis regulatory reform (the "Reform").
The new regulations under the Reform might remove many of the heavy regulations in the sector, making medical cannabis more accessible to patients as well as boosting export, all of which may materially and positively impact the business, financial
condition and results of operations of the Company, its subsidiaries and Focus. Due to the Israel-Hamas war, the anticipated implementation of the Reform, originally scheduled for December 29, 2023, has been postponed by three months.
On April 1, 2024, the Company announced the implementation of the medical cannabis regulatory reform in Israel on April 1st.
The Reform will be implemented in phases, as approved, and announced by the Israeli Ministry of Health. The key aspects of the
initial phase, commencing today, April 1st, are as follows:
1. Change in the prescription process: patients with a wide range of diseases and medical conditions from Oncology to Parkinsons
will no longer be required to obtain a license to receive medical cannabis. Patients will receive a prescription similar to those for other prescription medications. Pain and PTSD are not included in the Reform yet.
2. Medical cannabis will now be prescribed through the HMO's, Israel's public healthcare system: until the Reform, cannabis could
not be prescribed through the HMO's which cover the majority of the Israeli population.
3. The number of prescribing physicians is expected to increase: as of today, HMO physicians, who are dully trained and certified
within their field of expertise, can prescribe medical cannabis as a first line treatment, as opposed to a last resort, based on medical discretion for the approved indications.
4. The cost for prescription is anticipated to be reduced: the Ministry of Health limited the cost for a medical cannabis
prescription.
66
Management’s Discussion and Analysis
For the full report published by the Ministry of Health see (in Hebrew)- https://www.health.gov.il/hozer/mmk152_2016.pdf.
CCAA PROCEEDINGS
On September 14, 2023, a CCAA Termination Order was granted by the Honourable Justice Osborne (upon service on the Service List of an executed
certificate and the above CCAA Proceedings under the Companies Creditors’ Arrangement Act and the Stay Period were terminated without any further act or formality. On September 29th, 2023, Trichome Financial Corp. filed (or was deemed to have filed) an assignment (or a bankruptcy order was made against Trichome Financial Corp.), and Goldhar &
Associates Ltd., was appointed as trustee of the estate of the bankrupt by the official receiver (or the Court). The first meeting of creditors of the bankrupt was held on October 17th, 2023.
As a direct or indirect shareholder of the entities that make up the Trichome Group, the Company is subject to the priorities of other stakeholders in
the CCAA proceedings and will likely realize no return in the restructure of the Trichome Group business.
ENVIRONMENTAL RISKS
The Group’s operations are subject to environmental and occupational safety laws and regulations in certain jurisdictions, concerning, among other
things, emissions and discharges to water, air and land, the handling and disposal of hazardous and nonhazardous materials and wastes, and employee health and safety. The Group incurs ongoing costs and obligations related to compliance with
environmental and employee health and safety matters. Any failure to comply or maintain compliance with environmental and occupational safety laws and regulations may result in additional costs for corrective measures, penalties or restrictions on
manufacturing operations and could have a material adverse effect on the business, results of operations and financial condition of the Group.
RISKS INHERENT IN THE AGRICULTURAL BUSINESS
The Company’s business involves the growing of cannabis products by third party suppliers, which are agricultural products. As such, the business is
subject to the risks inherent in the agricultural business, such as pests, plant diseases and similar agricultural risks. Although, the third-party cultivators the Company partner with carefully monitor the growing conditions with trained personnel
and applicable equipment, there can be no assurance that natural elements will not have a material adverse effect on the production of its products and results of operations. Any decline in production could have a material adverse effect on the
Group’s business, operating results or financial condition.
Certain statements in this MD&A may contain “forward-looking statements” or “forward-looking information,” within the meaning of applicable
Canadian and United States securities legislation (collectively referred to herein as “forward-looking statements”). All statements other than statements of fact may be deemed to be forward-looking statements, including statements with regard to
expected financial performance, strategy and business conditions. The words “believe”, “plan”, “intend”, “estimate”, “expect”, “anticipate”, “continue”, or “potential”, and similar expressions, as well as future or conditional verbs such as “will”,
“should”, “would”, and “could” often identify forward-looking statements. These statements reflect management’s current expectations and plans with respect to future events and are based on information currently available to management including
based on reasonable assumptions, estimates, internal and external analysis and opinions of management considering its experience, perception of trends, current conditions and expected developments as well as other factors that management believes
to be relevant as at the date such statements are made. No assurance can be given that the expectations in any forward-looking statement will prove to be correct and, as such, the forward-looking statements included in this MD&A should not be
unduly relied upon. Forward-looking statements is by its nature prospective and requires IM Cannabis to make certain assumptions and is subject to inherent risks and uncertainties. All forward-looking statements are provided as of the date of this
MD&A. The Company does not undertake to update any such forward-looking statements whether as a result of new information, future events or otherwise, except as required by law.
67
Management’s Discussion and Analysis
Forward-looking statements in this MD&A may include, without limitation, forward-looking statements pertaining to:
| ● |
the Company’s business objectives and milestones and the anticipated timing of execution.
|
| ● |
the performance of the Company’s business, strategies and operations.
|
| ● |
the Company’s intentions to expand the business, operations and potential activities of the Company.
|
| ● |
the Company’s plans to expand its sales channels, distribution, delivery and storage capacity, and reach to medical cannabis patients.
|
| ● |
the competitive conditions of the cannabis industry and the growth of medical or adult-use recreational cannabis markets in the jurisdictions in which the Company operates.
|
| ● |
the competitive conditions of the industry, including the Company’s ability to maintain or grow its market share and maintain its competitive advantages.
|
| ● |
statements relating to the Company’s commitment to responsible growth and compliance with the strictest regulatory environments.
|
| ● |
the Company’s focus on providing premium cannabis products to medical patients in the jurisdictions in which the Company conducts business and any other jurisdiction in which the Company may conduct business in the future.
|
| ● |
the Company’s plans to amplify its commercial and brand power to become a global high-quality cannabis player.
|
| ● |
the Company’s primary goal of sustainably increasing revenue in its core markets.
|
| ● |
the demand and momentum in the Company’s Israeli and Germany operations.
|
| ● |
how the Company intends to position its brands.
|
| ● |
the efficiencies and synergies of the Company as a global organization with domestic expertise in Israel and Germany.
|
| ● |
expectations that providing high-quality, reliable supply to the Company’s customers and patients will lead to recurring sales.
|
| ● |
expectations related to the Company’s introduction of new Stock Keeping Unit (“SKUs”)
|
| ● |
anticipated cost savings from the reorganization of the Company's and the completion thereof upon the timelines disclosed herein.
|
| ● |
geographic diversification and brand recognition and the growth of the Company’s brands in the jurisdictions that the Company operates in or may expand to.
|
| ● |
expectations related to the Company’s ability to address the ongoing needs and preferences of medical cannabis patients.
|
| ● |
the Company’s retail presence, distribution capabilities and data-driven insights.
|
| ● |
the future impact of the Regulations Amendment regarding the transition reform from licenses to prescriptions for medical treatment of cannabis.
|
| ● |
the Company’s continued partnerships with third party suppliers and partners and the benefits thereof.
|
| ● |
the Company’s ability to achieve profitability in 2024.
|
| ● |
the number of patients in Israel licensed by the Israeli Ministry of Health (“MOH”) to consume medical cannabis.
|
68
Management’s Discussion and Analysis
| ● |
expectations relating to the number of patients paying out-of-pocket for medical cannabis products in Germany.
|
| ● |
the anticipated decriminalization or legalization of adult-use recreational cannabis in Israel and Germany.
|
| ● |
expectations related to the demand and the ability of the Company to source premium and ultra-premium cannabis products exclusively and competition in this product segment.
|
| ● |
the anticipated impact of inflation and liquidity on the Company’s performance.
|
| ● |
expectations with respect to the Company’s operating budget and the assumptions related thereto.
|
| ● |
expectations relating to the Company as a going concern and its ability to conduct business under the ordinary course of operations.
|
| ● |
expectations related to the collection the payment awarded in the Judgment and the chances of the claim advancing or the potential outcome of the Test Kits Appeal (as defined herein);
|
| ● |
the continued listing of the Company’s Common Shares in the capital of the Company (“Common Shares”) on the Nasdaq Stock Market (“Nasdaq”) and Canadian
Securities Exchange (“CSE”);
|
| ● |
cannabis licensing in the jurisdictions in which the Company operates.
|
| ● |
the renewal and/or extension of the Company’s licenses.
|
| ● |
the Company’s anticipated operating cash requirements and future financing needs.
|
| ● |
the Company’s expectations regarding its revenue, expenses, profit margins and operations.
|
| ● |
the anticipated Gross Margins, EBITDA and Adjusted EBITDA from the Company’s operations.
|
| ● |
the expected increase in revenue and margins in its Israeli medical cannabis market activities arising from its acquisitions.
|
| ● |
future opportunities for the Company in Israel, particularly in the retail and distribution segments of the cannabis market.
|
| ● |
future expansion and growth opportunities for the Company in Germany and Europe and the timing of such.
|
| ● |
contractual obligations and commitments; and
|
| ● |
the Company completing the Potential Transaction with Kadimastem (each as defined herein).
|
With respect to the forward-looking statements contained in this MD&A, the Company has made assumptions regarding, among other things:
| ● |
the Company has the ability to achieve its business objectives and milestones under the stated timelines.
|
| ● |
the Company will succeed in carrying out its business, strategies and operations.
|
| ● |
the Company will realize upon its intentions to expand the business, operations and potential activities of the Company.
|
| ● |
the Company will expand its sales channels, distribution, delivery and storage capacity, and reach to medical cannabis patients.
|
| ● |
the competitive conditions of the cannabis industry and the growth of medical or adult-use recreational cannabis in the jurisdictions in which the Company operates.
|
| ● |
the competitive conditions of the industry will be favourable to the Company, and the Company has the ability to maintain or grow its market share and maintain its competitive advantages.
|
| ● |
the Company will commit to responsible growth and compliance with the strictest regulatory environments.
|
69
Management’s Discussion and Analysis
| ● |
the Company will remain focused on providing premium cannabis products to medical patients in the jurisdictions in which the Company conducts business and any other jurisdiction in which the Company may conduct business in the future.
|
| ● |
the Company has the ability to amplify its commercial and brand power to become a global high-quality cannabis player.
|
| ● |
the Company will maintain its primary goal of sustainably increasing revenue in its core markets.
|
| ● |
the demand and momentum in the Company’s Israeli and Germany operations will be favourable to the Company.
|
| ● |
the Company will carry out its plans to position its brands as stated.
|
| ● |
the Company’s Company has the ability to realize upon the stated efficiencies and synergies the Company as a global organization with domestic expertise in Israel and Germany.
|
| ● |
providing a high-quality, reliable supply to the Company’s customers and patients will lead to recurring sales.
|
| ● |
the Company will introduce new SKUs.
|
| ● |
the Company has the ability to achieve geographic diversification and brand recognition and the growth of the Company’s brands in the jurisdictions that the Company operates in or may expand to.
|
| ● |
the Company’s has the ability to address the ongoing needs and preferences of medical cannabis patients;
|
| ● |
the Company has the ability to realize upon its retail presence, distribution capabilities and data-driven insights.
|
| ● |
the future impact of the Regulations Amendment will be favourable to the Company.
|
| ● |
the Company will maintain its partnerships with third parties, suppliers and partners.
|
| ● |
the Company has the ability to achieve profitability in 2024;
|
| ● |
the accuracy of number of patients in Israel licensed by the MOH to consume medical cannabis.
|
| ● |
the accuracy of the number of patients paying out-of-pocket medical cannabis products in Germany.
|
| ● |
the anticipated decriminalization or legalization of adult-use recreational cannabis in Israel and Germany will occur.
|
| ● |
the Company has the ability to source premium and ultra-premium cannabis products exclusively and competition in this product segment.
|
| ● |
the anticipated impact of inflation and liquidity on the Company’s performance will be as forecasted.
|
| ● |
the accuracy with respect to the Company’s operating budget and the assumptions related thereto.
|
| ● |
the Company will remain as going concern.
|
| ● |
a favourable outcome with respect to the collection the payment awarded in the Judgment and the chances of the claim advancing or the potential outcome of the Test Kits Appeal.
|
| ● |
the Company’s Common Shares will remain listed on the Nasdaq and the CSE.
|
| ● |
the Company’s ability to maintain cannabis licensing in the jurisdictions in which the Company operates.
|
| ● |
the Company has the ability to obtain the renewal and/or extension of the Company’s licenses.
|
| ● |
the Company has the ability to meet operating cash requirements and future financing needs.
|
| ● |
the Company will meet or surpass its expectations regarding its revenue, expenses, profit margins and operations.
|
70
Management’s Discussion and Analysis
| ● |
the Company will meet or surpass its expectations regarding Gross Margins, EBITDA and Adjusted EBITDA from the Company’s operations.
|
| ● |
the Company will increase its revenue and margins in its Israeli medical cannabis market activities arising from its acquisitions.
|
| ● |
the Company has the ability to capitalize on future opportunities for the Company in Israel, particularly in the retail and distribution segments of the cannabis market.
|
| ● |
the Company will carry out its future expansion and growth opportunities for the Company in Germany and Europe and the timing of such.
|
| ● |
the Company will fulfill its contractual obligations and commitments; and
|
| ● |
the Company will complete the Proposed Transaction with Kadimastem.
|
Readers are cautioned that the above lists of forward-looking statements and assumptions are not exhaustive. Since forward-looking statements address future events and conditions, by their very nature they involve inherent risks and uncertainties. Actual results may differ materially from those currently anticipated or implied by such forward-looking statements due to a number of factors and risks. These include:
| ● |
the Company’s inability to achieve its business objectives and milestones under the stated timelines.
|
| ● |
the Company inability to carry out its business, strategies and operations.
|
| ● |
the Company’s inability to realize upon its intentions to expand the business, operations and potential activities of the Company.
|
| ● |
the Company will not expand its sales channels, distribution, delivery and storage capacity, and reach to medical cannabis patients.
|
| ● |
the competitive conditions of the cannabis industry and the growth of medical or adult-use recreational cannabis markets will be unfavourable to the Company in the jurisdictions in which the Company operates.
|
| ● |
the competitive conditions of the industry will be unfavourable to the Company, and the Company’s inability to maintain or grow its market share and maintain its competitive advantages.
|
| ● |
the Company will not commit to responsible growth and compliance with the strictest regulatory environments.
|
| ● |
the Company’s inability to remain focused on providing premium cannabis products to medical patients in the jurisdictions in which the Company conducts business and any other jurisdiction in which the Company may conduct business in the
future.
|
| ● |
the Company inability to amplify its commercial and brand power to become a global high-quality cannabis player.
|
| ● |
the Company will not maintain its primary goal of sustainably increasing revenue in its core markets.
|
| ● |
the demand and momentum in the Company’s Israeli and Germany operations will be unfavourable to the Company.
|
| ● |
the Company will not carry out its plans to position its brands as stated.
|
| ● |
the Company’s inability to realize upon the stated efficiencies and synergies of the Company as a global organization with domestic expertise in Israel and Germany.
|
| ● |
providing a high-quality, reliable supply to the Company’s customers and patients will not lead to recurring sales.
|
| ● |
the Company will not introduce of new SKUs.
|
| ● |
the Company’s inability to realize upon the anticipated cost savings from the reorganization.
|
71
Management’s Discussion and Analysis
| ● |
the Company’s inability to achieve geographic diversification and brand recognition and the growth of the Company’s brands in the jurisdictions that the Company operates in or may expand to.
|
| ● |
the Company’s inability to address the ongoing needs and preferences of medical cannabis patients.
|
| ● |
the Company’s inability to realize upon its retail presence, distribution capabilities and data-driven insights.
|
| ● |
the future impact of the Regulations Amendment will be unfavourable to the Company.
|
| ● |
the Company will not maintain its partnerships with third party suppliers and partners.
|
| ● |
the inaccuracy of number of patients in Israel licensed by the MOH to consume medical cannabis.
|
| ● |
the inaccuracy of the number of patients paying out-of-pocket for medical cannabis products in Germany.
|
| ● |
the anticipated decriminalization or legalization of adult-use recreational cannabis in Israel and Germany will not occur.
|
| ● |
the Company’s ability to source premium and ultra-premium cannabis products exclusively and competition in this product segment.
|
| ● |
the anticipated impact of inflation and liquidity on the Company’s performance will not be as forecasted.
|
| ● |
the inaccuracy with respect to the Company’s operating budget and the assumptions related thereto.
|
| ● |
the Company will not remain as going concern.
|
| ● |
an unfavourable outcome of the negotiations or the Construction Proceedings.
|
| ● |
an unfavourable outcome with respect to the collection the payment awarded in the Judgment and the chances of the claim advancing or the potential outcome of the Appeal.
|
| ● |
the Company’s Common Shares will not remain listed on the Nasdaq and the CSE.
|
| ● |
the Company’s inability to maintain cannabis licensing in the jurisdictions in which the Company operates.
|
| ● |
the Company’s inability to obtain the renewal and/or extension of the Company’s licenses.
|
| ● |
the Company’s inability to meet operating cash requirements and future financing needs.
|
| ● |
the Company will not meet or surpass its expectations regarding its revenue, expenses, profit margins and operations.
|
| ● |
the Company will not meet or surpass its expectations regarding Gross Margins, EBITDA and Adjusted EBITDA from the Company’s operations.
|
| ● |
the Company will not increase its revenue and margins in its Israeli medical cannabis market activities arising from its acquisitions.
|
| ● |
the Company’s ability to capitalize on future opportunities for the Company in Israel, particularly in the retail and distribution segments of the cannabis market.
|
| ● |
the Company will not carry out its future expansion and growth opportunities for the Company in Germany and Europe and the timing of such; and
|
| ● |
the Company will not fulfill its contractual obligations and commitments; and
|
Readers are cautioned that the foregoing list of risk factors is not exhaustive. Additional information on these and other factors that could affect the business, operations or financial results of the Company are detailed under the headings “Risk and Factors” and “Contingent Liabilities and Commitments” of this MD&A. The Company and management caution readers not to place undue reliance on any forward-looking statements, which speak only as of the date made. Although the Company believes that the expectations reflected in the forward-looking statements are reasonable, it can give no assurance that such expectations will prove to have been correct. The Company and management assume no obligation to update or revise them to reflect new events or circumstances except as required by applicable securities laws.
Additional information about the assumptions, risks and uncertainties of the Company’s business and material factors or assumptions on which
information contained in forward-looking statements is based is provided in the Company’s disclosure materials, including in this MD&A under “Legal and Regulatory – Risk Factors” and the Company’s Annual
Report under “Risk Factors”, available on the Company’s profile on SEDAR+ at www.sedarplus.ca and on EDGAR at www.sec.gov/edgar.
All forward-looking statements in this MD&A is qualified by these cautionary statements.
ADDITIONAL INFORMATION
Additional information relating to the Company, including the Company’s Annual Report, is available on the Company’s profile on SEDAR+ at www.sedarplus.ca and on
EDGAR at www.sec.gov/edgar.
- - - - - - - - - - - - - - - - - - - - - -
72