IM Cannabis Corp.
Management’s Discussion and Analysis
For the
Three and Nine Months Ended September 30, 2021
November
15, 2021
IM
Cannabis Corp.
Management’s Discussion and Analysis (Canadian dollars, in
thousands)
IM Cannabis Corp.
Management’s Discussion and Analysis
For the Three and Nine Months Ended September 30, 2021 and
2020
This
Management’s Discussion and Analysis (“MD&A”)
reports on the consolidated financial condition and operating
results of IM Cannabis Corp. (the “Company” or
“IMCC”) for the three and nine months ended September
30, 2021 and 2020. Throughout this MD&A, unless otherwise
specified, references to “we”, “us”,
“our” or similar terms, as well as the
“Company” and “IMCC” refer to IM Cannabis
Corp., together with its subsidiaries, on a consolidated basis, and
the “Group” refers to the Company, its subsidiaries and
Focus Medical Herbs Ltd.
This
MD&A should be read in conjunction with the Company’s
interim condensed consolidated financial statements for the three
and nine months ended September 30, 2021 (the “Interim
Financial Statements”) and with the Company’s annual
financial statements as of December 31, 2020, and for the year then
ended and accompanying notes (“Annual Financial
Statements”).
The
Interim Financial Statements have been prepared by management in
accordance with the International Financial Reporting Standards
(“IFRS”) as issued by the International Accounting
Standards Board (“IASB”). IFRS requires management to
make certain judgments, estimates and assumptions that affect the
reported amount of assets and liabilities at the date of the
financial statements and the amount of revenue and expenses
incurred during the reporting period. The results of operations for
the periods reflected herein are not necessarily indicative of
results that may be expected for future periods.
The
Interim Financial Statements include the accounts of the Company,
and the following entities:
|
Legal Entity:
|
Relationship with the Company:
|
|
IMC
Holdings Ltd. (“IMC Holdings”)
|
Wholly-owned
subsidiary
|
|
Adjupharm
GmbH (“Adjupharm”)
|
Subsidiary
of IMC Holdings
|
|
IMC
Ventures Ltd.
|
Subsidiary
of IMC Holdings
|
|
I.M.C
Farms Israel Ltd.
|
Wholly-owned
subsidiary of IMC Holdings
|
|
I.M.C.
– International Medical Cannabis Portugal Unipessoal,
Lda.
|
Wholly-owned
subsidiary of IMC Holdings
|
|
Focus
Medical Herbs Ltd. (“Focus”)
|
Private
company over which IMC Holdings exercises “de facto
control” under IFRS 10, as further described under the
“Risk Factors” section below
|
|
R.A.
Yarok Pharm Ltd. (“Pharm Yarok”)
|
Private
company over which IMC Holdings exercises control under accounting
principles, as further described under the “Overview of the Company– The
Group and Consolidated Entities – The Consolidated
Entities” section below
|
IM
Cannabis Corp.
Management’s Discussion and Analysis (Canadian dollars, in
thousands)
|
Rosen
High Way Ltd. (“Rosen High Way”)
|
Private
company over which IMC Holdings exercises control under accounting
principles, as further described under the “Overview of the Company– The
Group and Consolidated Entities – The Consolidated
Entities” section below
|
|
High
Way Shinua Ltd. (“HW Shinua”)
|
Private
company over which IMC Holdings exercises control under accounting
principles, as further described under the “Overview of the Company– The
Group and Consolidated Entities – The Consolidated
Entities” section below
|
|
Revoly
Trading and Marketing Ltd. dba Vironna Pharm
(“Vironna”)
|
Private
company over which IMC Holdings exercises control under accounting
principles, as further described under the “Overview of the Company– The
Group and Consolidated Entities – The Consolidated
Entities” section below
|
|
Trichome
Financial Corp. (“Trichome”)
|
Wholly-owned
subsidiary
|
|
Trichome
Financial Cannabis GP Inc.
|
Wholly-owned
subsidiary of Trichome
|
|
Trichome
Financial Cannabis Manager Inc.
|
Wholly-owned
subsidiary of Trichome
|
|
Trichome
Financial Cannabis Private Credit LP (the
“Fund”)
|
Limited
partnership, equity accounted investee
|
|
Trichome
Asset Funding Corp.
|
Wholly-owned
subsidiary of Trichome
|
|
Trichome
JWC Acquisition Corp. (“TJAC”)
|
Wholly-owned
subsidiary of Trichome
|
|
Trichome
Retail Corp.
|
Wholly-owned
subsidiary of Trichome
|
|
MYM
Nutraceuticals Inc. (“MYM”)
|
Wholly-owned
subsidiary of Trichome
|
|
SublimeCulture
Inc.
|
Wholly-owned
subsidiary of MYM
|
|
CannaCanada
Inc.
|
Wholly-owned
subsidiary of MYM
|
|
MYM
International Brands Inc.
|
Wholly-owned
subsidiary of MYM
|
|
Highland
Grow Inc.
|
Wholly-owned
subsidiary of MYM International Brands Inc.
|
All
intercompany balances and transactions were eliminated on
consolidation.
All
amounts in this MD&A are expressed in Canadian Dollars ($) in
thousands, unless otherwise noted. Certain amounts are shown in New
Israeli Shekel (“NIS”), Euro (“€”),
United States Dollars (“US$”) as$”), in
thousands, unless otherwise noted.
CAUTION CONCERNING FORWARD-LOOKING STATEMENTS
Certain
statements in this MD&A may contain “forward-looking
statements” or “forward-looking information,”
within the meaning of applicable securities legislation
(collectively referred to herein as “forward-looking
statements” or “forward-looking information”).
All statements other than statements of fact may be deemed to be
forward-looking statements, including statements with regard to
expected financial performance, strategy and business conditions.
The words “believe”, “plan”,
“intend”, “estimate”, “expect”,
“anticipate”, “continue”, or
“potential”, and similar expressions, as well as future
or conditional verbs such as “will”,
“should”, “would”, and “could”
often identify forward-looking statements. These statements reflect
management’s current expectations and plans with respect to
future events and are based on information currently available to
management including based on reasonable assumptions, estimates,
internal and external analysis and opinions of management
considering its experience, perception of trends, current
conditions and expected developments as well as other factors that
management believes to be relevant as at the date such
statements are
made.
IM
Cannabis Corp.
Management’s Discussion and Analysis (Canadian dollars, in
thousands)
Without
limitation, this MD&A contains forward-looking statements
pertaining to:
●
the Company’s
business objectives and milestones and the anticipated timing of
execution;
●
the performance of
the Company’s business and operations;
●
the intention to
expand the business, operations and potential activities of the
Company;
●
expectations
relating to the number of patients in Israel licensed by the
Israeli Ministry of Health (“MOH”) to consume medical
cannabis;
●
expectations of
Focus, TJAC and MYM on variations of net cost of sales based on the
number of pre-harvest plants, after harvest plants, the strains
being grown and technological progress in the trimming
machines;
●
the future product
portfolios of the Group and the Company’s ability to export
its products, strains and genetics from Canada to Israel and
Germany;
●
the competitive
conditions of the cannabis industry and the growth of medical or
recreational cannabis markets in the jurisdictions in which the
Company operates;
●
the growth of the
Company’s brands in the respective
jurisdictions;
●
the Company’s
retail presence, distribution capabilities and data-driven
insights;
●
cannabis licensing
in Israel, Germany and Canada, including the anticipated
decriminalization or legalization of recreational cannabis in
Israel;
●
expectations
regarding the renewal and/or extension of the Group’s
licenses;
●
the Group’s
anticipated operating cash requirements and future financing
needs;
●
the Group’s
expectations regarding its revenue, expenses, profit margins and
operations;
●
future
opportunities for the Company in Canada, particularly in the
premium and ultra-premium segments;
●
future
opportunities for the Company in Israel, particularly in the retail
and distribution segments of the cannabis market; and
●
contractual
obligations and commitments.
With
respect to the forward looking-statements contained in this
MD&A, the Company has made assumptions regarding, among other
things:
●
the anticipated
increase in demand for medical and recreational cannabis in the
markets in which the Company operates;
●
the Company’s
satisfaction of international demand for its products;
●
the effectiveness
of its products for medical cannabis patients and recreational
consumers;
●
future cannabis
pricing and input costs;
●
cannabis production
yields;
●
the Company being
able to continue to drive organic growth from Canadian operations;
and
●
the Company’s
ability to market its brands and services successfully to its
anticipated customers.
Readers
are cautioned that the above lists of forward-looking statements
and assumptions are not exhaustive. Since forward-looking
statements address future events and conditions, by their very
nature they involve inherent risks and uncertainties. Actual
results may differ materially from those currently anticipated or
implied by such forward-looking statements due to a number of
factors and risks. These include:
IM
Cannabis Corp.
Management’s Discussion and Analysis (Canadian dollars, in
thousands)
●
general business
risk and liability, including claims or complaints in the normal
course of business;
●
any failure of the
Company to maintain “de facto” control over Focus in
accordance with IFRS 10 Consolidated Financial Statements
(“IFRS 10”);
●
regulatory
authorities in Israel viewing the Company as the deemed owner of
more than 5% of Focus in contravention of Israeli
regulations;
●
limitations on
stockholdings of the Company in connection with its potential
direct engagement in the Israeli medical cannabis
market;
●
the ability and/or
need to obtain additional financing for continued
operations;
●
the lack of control
over the Company’s investees;
●
the failure of the
Company to comply with applicable regulatory requirements in a
highly regulated industry;
●
unexpected changes
in governmental policies and regulations affecting the production,
distribution, manufacture or use of medial cannabis in Israel,
Germany, Canada, Portugal, Greece, or any jurisdictions in which
the Company intends to operate;
●
the Company’s
ability to continue to meet the listing requirements of the
Canadian Securities Exchange (“CSE”) and the NASDAQ
Capital Market (“NASDAQ”);
●
the Israeli
government deciding to abandon the decriminalization or
legalization of recreational cannabis;
●
any change in the
political environment which would negatively affect the prospect of
decriminalization or legalization of recreational cannabis in
Israel;
●
any unexpected
failure of Focus to maintain in good standing or renew any of the
licenses granted by the Israeli Medical Cannabis Agency (the
“IMCA”), administered by the MOH, to propagate and
cultivate medical cannabis in Israel (the “Focus
Licenses”);
●
Focus’
reliance on the Focus Facility (as defined herein) to conduct
medical cannabis activities in Israel;
●
any unexpected
failure of Focus to maintain the Focus Facility in good standing
with all state and municipal Israeli regulations, including all
required licenses and permits and under the Focus
Leases;
●
any adverse outcome
of the Construction Proceedings (as defined herein);
●
any unexpected
failure of Adjupharm to maintain in good standing or renew any of
its wholesale, narcotics handling, manufacturing, procurement,
storage and distribution, or import/export licenses, permits,
certificates or approvals granted to Adjupharm by German regulatory
authorities (the “Adjupharm Licenses”);
●
any unexpected
failure of TJAC to maintain in good standing or renew any of the
Canadian licenses held by TJAC to produce, process, and sell
cannabis products in the adult-use recreational cannabis market
(the “TJAC Licenses”) or the Canadian licenses held by
MYM to cultivate, process, and sell cannabis products in the
adult-use recreational cannabis market (the “MYM
Licenses”);
●
the reliance by
TJAC and MYM on their facilities to conduct medical cannabis
activities;
●
any unexpected
failure of TJAC and/or MYM to maintain their facilities in good
standing with all applicable regulations, including all required
licenses and permits and under the TJAC Leases and the Sublime
Lease;
●
the Group’s
ability to maintain ancillary business licenses, permits and
approvals required to operate effectively;
●
the ability to
complete the Company’s acquisition of certain trading house
and in-house pharmacy assets from Panaxia Pharmaceutical Industries
Israel Ltd. and Panaxia Logistics Ltd., part of the Panaxia Labs
Israel, Ltd. group of companies (“Panaxia”) (the
“Panaxia Transaction”) in a timely manner or at all,
including (i) the receipt of requisite MOH approval in connection
with the transfer of Panaxia’s IMC-GDP license (the
“Panaxia IMC-GDP License”); (ii) any exercise of the
Company’s option (the “Panaxia Option”) to
acquire Panaxia’s pharmacy and its licenses to dispense and
sell medical cannabis to licensed medical cannabis patients (the
“Panaxia Pharmacy Licenses”); and (iii) the timing of
each instalment of the share consideration component of the Panaxia
Transaction purchase price (“Panaxia Consideration
Shares”);
IM
Cannabis Corp.
Management’s Discussion and Analysis (Canadian dollars, in
thousands)
●
the ability to
complete the acquisition of the outstanding ordinary shares of
Pharm Yarok, Rosen High Way and HW Shinua (Collectively, the
“Pharm Yarok Group”) (the “Pharm Yarok
Transaction”), including the receipt of MOH approval in
connection with the change of control under their respective
licenses of cannabis activity in Israel following the Pharm Yarok
Transaction;
●
the ability to
complete the acquisition of 51% of the outstanding ordinary shares
of Vironna (the “Vironna Transaction”), including the
receipt of MOH approval in connection with the change of control
under its licenses to dispense and sell medical cannabis to
licensed medical cannabis patients;
●
the ability of the
Company, following the Trichome Transaction, the MYM Transaction
(as defined herein), the Panaxia Transaction, the Pharm Yarok
Transaction and the Vironna Transaction to integrate each entity
into the Company’s operations and realize the anticipated
benefits and synergies of each such transaction and the timing
thereof and the focus of management on such
integration;
●
any potential
undisclosed liabilities of Trichome, MYM, Pharm Yarok, Vironna or
other entities acquired by the Company that were unidentified
during the due diligence process;
●
the interpretation
of Company’s acquisitions of companies or assets by tax
authorities or regulatory bodies, including but not limited to the
change of control of licensed entities;
●
the failure to
negotiate and execute a definitive agreement with cbdMD, Inc.
(“cbdMD”) satisfactory to the respective
parties;
●
the ability of the
Group to deliver on their sales commitments or growth
objectives;
●
the Group’s
reliance on third-party supply agreements and its ability to enter
into additional supply agreements to provide sufficient quantities
of medical cannabis to fulfil the Group’s
obligations;
●
the Group’s
possible exposure to liability, the perceived level of risk related
thereto, and the anticipated results of any litigation or other
similar disputes or legal proceedings involving the Group,
including but not limited to the Construction Proceedings, the MOH
Allegations (each as defined herein) and the class action
proceedings described herein;
●
the impact of
increasing competition;
●
any lack of merger
and acquisition opportunities;
●
inconsistent public
opinion and perception regarding the use of cannabis;
●
engaging in
activities considered illegal under US federal law related to
cannabis;
●
political
instability and conflict in the Middle East;
●
adverse market
conditions;
●
unexpected
disruptions to the operations and businesses of the Group as a
result of the COVID-19 global pandemic or other disease outbreaks
including a resurgence in the cases of COVID-19;
●
the inherent
uncertainty of production quantities, qualities and cost estimates
and the potential for unexpected costs and expenses;
●
the Group’s
ability to sell its products
●
any change in
accounting practices or treatment affecting the consolidation of
financial results;
●
reliance on
management; and
●
the loss of key
management and/or employees.
IM
Cannabis Corp.
Management’s Discussion and Analysis (Canadian dollars, in
thousands)
Readers
are cautioned that the foregoing list of risk factors is not
exhaustive. Additional information on these and other factors that
could affect the business, operations or financial results of the
Company are detailed under the headings “Risks Factors”
and “Contingent Liabilities and Commitments” of this
MD&A. The Company and management caution readers not to place
undue reliance on any forward-looking statements, which speak only
as of the date made. Although the Company believes that the
expectations reflected in the forward-looking statements are
reasonable, it can give no assurance that such expectations will
prove to have been correct. The Company and management assume no
obligation to update or revise them to reflect new events or
circumstances except as required by applicable securities
laws.
FINANCIAL OUTLOOK
The
forward-looking information in this MD&A contain statements in
respect of estimated revenues. The Company and its management
believe that the estimated revenues are reasonable as of the date
hereof and are based on management’s current views,
strategies, expectations, assumptions and forecasts, and have been
calculated using accounting policies that are generally consistent
with the Company’s current accounting policies. These
estimates are considered financial outlooks under applicable
securities legislation. These estimates and any other financial
outlooks or future-oriented financial information included herein
have been approved by management of the Company as of the date
hereof. Such financial outlooks or future-oriented financial
information are provided for the purposes of presenting information
about management’s current expectations and goals relating to
the sales agreements described in the “Corporate
Developments” section of this MD&A and other previously
announced Focus sales agreements and the future business of the
Company. The Company disclaims any intention or obligation to
update or revise any future-oriented financial information, whether
as a result of new information, future events or otherwise, except
as required by securities legislation. Readers are cautioned that
actual results may vary materially as a result of a number of
risks, uncertainties, and other factors, many of which are beyond
the Group’s control. See the risks and uncertainties
discussed in the “Risk Factors” section and elsewhere
in this MD&A and other risks detailed from time to time in the
publicly filed disclosure documents of the Company which can be
viewed online under the Company’s profile on the System for
Electronic Document Analysis and Retrieval (“SEDAR”) at
www.sedar.com.
NON-IFRS FINANCIAL MEASURES
Certain
financial measures used in this MD&A do not have any
standardized meaning under IFRS, including “Gross
Margin”, “EBITDA” and “Adjusted
EBITDA”. For a reconciliation of these non-IFRS financial
measures to the most comparable IFRS financial measures, as
applicable, see the “Metrics and Non-IFRS Financial
Measures” section of the MD&A.
OVERVIEW
OF THE COMPANY
Company Background
The
Company was incorporated pursuant to the Business Corporations Act (British
Columbia) on March 7, 1980, under the name “Nirvana Oil &
Gas Ltd.” On July 12, 2013, in connection with a share
consolidation, the Company changed its name to “Navasota
Resources Inc.”. On June 22, 2018, the Company completed a
consolidation of its common shares (“Common Shares”) on
the basis of one post-consolidation Common Share for every five
pre-consolidation Common Shares. On October 4, 2019, in connection
with the Reverse Takeover Transaction (as defined below), the
Company effected a consolidation of its Common Shares on the basis
of one (1) post-consolidation Common Share for every 2.83
pre-consolidation Common Shares and changed its name to “IM
Cannabis Corp.”. On October 11, 2019, the Company completed
the Reverse Takeover Transaction and changed its business from
mining to the international medical cannabis industry.
IM
Cannabis Corp.
Management’s Discussion and Analysis (Canadian dollars, in
thousands)
On
February 12, 2021, in connection with its NASDAQ listing
application, the Company effected a consolidation of its Common
Shares on the basis of one (1) post-consolidation Common Share for
every four (4) pre-consolidation Common Shares.
IMCC is
a multi-country operator in the medical and recreational cannabis
sector headquartered in Israel with operations in Israel, Europe,
and Canada.
Israel
In
Israel, IMC Holdings built the IMC brand of premium medical
cannabis products which have been cultivated over the last decade
by Focus, an Israeli licensed cultivator over which IMC Holdings
exercises “de facto control” under IFRS 10, and its
cultivation partners, and sold by Focus in the Israeli
market.
Focus
holds the Focus Licenses, granted by the MOH, to propagate and
cultivate medical cannabis in the State of Israel, valid until
January 3, 2022s. Focus is one of the eight medical cannabis
producers initially licensed by Israeli regulatory authorities and
has over 10 years of experience in growing high quality medical
cannabis products for the Israeli market.
As part
of its core Israeli business, IMC Holdings offers intellectual
property-related services to the medical cannabis industry based on
proprietary processes and technologies it developed for the
production of medical cannabis. The Company offers its intellectual
property and consulting services to Focus pursuant to certain
commercial agreements and receives as consideration for such
services a share of Focus’ revenues resulting from the sale
of medical cannabis products under the IMC brand. The Company has
started entering, through its subsidiaries, the distribution and
retail segments of the Israeli medical cannabis market, by entering
into each of the Panaxia Transaction, the Pharm Yarok Transaction,
and the Vironna Transaction and by attracting acquisitions of
synergistic targets in Israel. Following such vertical integration,
IMCC expects to increase its revenues from its Israeli medical
cannabis market activities, diversify its business opportunities
and gain direct access to medical cannabis patients.
Europe
In
Europe, IMCC operates through Adjupharm, a German-based subsidiary
acquired by IMC Holdings on March 15, 2019. Adjupharm is an EU-GMP
certified medical cannabis producer and distributor with the
Adjupharm Licenses that allow, among other capabilities, for the
import/export of medical cannabis with requisite permits. Adjupharm
serves as the Company’s flagship European outpost for sales
and distribution.
Adjupharm
currently manufactures and distributes IMC-branded medical cannabis
products, in addition to other branded medical cannabis products,
to pharmacies and distribution partners in Germany pursuant to
sales and distribution agreements. Adjupharm sources its medical
cannabis products from strategic partners, including various EU-GMP
standard European and Canadian suppliers. While the Company does
not currently distribute products in European countries other than
Germany, the Company intends to leverage the platform established
by Adjupharm in Germany and its network of distribution partners to
expand to other jurisdictions across the continent in which medical
cannabis is legal.
IM
Cannabis Corp.
Management’s Discussion and Analysis (Canadian dollars, in
thousands)
Canada
IMCC
expanded operations to Canada through the acquisition of Trichome
on March 18, 2021 with the objective of securing premium indoor
cannabis for the Israeli medical market as well as to compete in
the premium segment of Canada’s adult-use recreational
market. Trichome’s wholly-owned subsidiary, TJAC holds the
TJAC Licenses. TJAC’s flagship facility, located near
Toronto, cultivates high-quality indoor cannabis using roughly
47,000 square feet of cultivation space. The Company intends to
operate Trichome with a focus on acquiring, integrating and
managing related assets in Canada that complement IMCC’s
international strategic objectives.
On July
9, 2021, IMCC, through Trichome, completed the acquisition of MYM
(the “MYM Transaction”). MYM is a Canadian cultivator,
processor, and distributor of premium cannabis through its two
wholly-owned subsidiaries: SublimeCulture Inc.
(“Sublime”), which is located near Montreal, Quebec,
and Highland Grow Inc. (“Highland”), which is in
Antigonish, Nova Scotia. MYM’s flagship brand, Highland, is
an ultra-premium brand sold in most provinces and territories
throughout Canada.
The
consolidated revenues of the Group for the three and nine months
ended September 30, 2021, was generated mainly from the sale of
medical cannabis products in Israel and Germany, and since March
18, 2021, the sale of adult-use recreational cannabis in Canada.
..
The
Group does not engage in any U.S. cannabis-related activities as
defined in Canadian Securities Administrators Staff Notice 51-352
(Revised) – Issuers with U.S. Marijuana-Related
Activities.
The Group and Consolidated Entities
The Group
As of
September 30, 2021, the Company’s major Israeli assets
include the Commercial Agreements and the Focus Agreement (as
defined herein), the respective definitive agreements with each of
the Consolidated Entities (as defined herein), as well as its
holdings in Xinteza API Ltd. (“Xinteza”).
As of
September 30, 2021, the Company’s major international assets
include Trichome, TJAC and MYM in Canada, and 90.02% of Adjupharm
in Germany.
As of
September 30, 2021, neither the Company nor any of its subsidiaries
currently hold, directly or indirectly, any licenses to engage in
the propagation, cultivation, production, processing, distribution
or sale of medical cannabis products in Israel as required by local
legislation. However, under IFRS 10, the Company is required to
consolidate the results of Focus, a licensed propagator and
cultivator of medical cannabis products under the current Israeli
regulatory regime. As such, all financial information in this
MD&A is presented on a consolidated basis reflecting the
results of the Group. While, as of the date of this report, IMCC
does not hold any of the Israeli licenses mentioned above and does
not own Focus, it derives a significant portion of its consolidated
revenues from Focus’ revenue, which is primarily earned from
sales of medical cannabis by Focus to pharmacies in Israel.
Furthermore, the Company has an option under the Focus Agreement
(as defined herein) to re-acquire 74% ownership of Focus. For more
information, please see the “Risk Factors” section
below.
IM
Cannabis Corp.
Management’s Discussion and Analysis (Canadian dollars, in
thousands)
The Consolidated Entities
On July
28, 2021, IMC Holdings entered into a definitive agreement in
respect of the Pharm Yarok Transaction, whereby it will acquire all
of the outstanding ordinary shares of Pharm Yarok Group, subject to
MOH approval.
On
August 18, 2021, IMC Holdings entered into a definitive agreement
in respect of the Vironna Transaction, whereby it will acquire 51%
of the outstanding ordinary shares of Vironna, subject to MOH
approval.
Although
IMC Holdings has entered into definitive agreements in respect of
each of the Pharm Yarok Transaction and the Vironna Transaction, it
has not yet completed the foregoing acquisitions. As such, IMC
Holdings does not own any of Pharm Yarok Group or Vironna
(collectively, the “Consolidated Entities”) or their
respective licenses to conduct cannabis-related activities in
Israel. However, the respective definitive agreements with each of
the Consolidated Entities, provides the Company with the power to
unilaterally make all decisions regarding the financial and
operating policies of each of the Consolidated Entities and the
rights to obtain all related economic benefits. Accordingly, under
IFRS, the Company consolidates the financial results of the
Consolidated Entities in the Interim Financial Statements
commencing on the date of signing of each of the respective
definitive agreements and, accordingly, the relevant financial
information in this MD&A is presented on a consolidated basis
reflecting the results of the Group and the Consolidated Entities.
For more information, please see the “Risk Factors”
section below.
Company Products
Israel
‘IMC’
is a well-known medical cannabis brand in Israel. Leveraging its
long-term success in the Israeli market, the Company launched the
IMC brand in Germany in 2020. The Company believes that the IMC
brand has become synonymous with quality and consistency in the
Israeli medical cannabis market. In August 2020, a survey of
licensed medical cannabis patients showed that the IMC brand is one
of the top four most popular medical cannabis brands in
Israel.1
In
association with Focus, the Company maintains a brand portfolio
that includes popular medical cannabis dried flowers such as Roma,
DQ, London, and Tel Aviv, as well as full-spectrum cannabis
extracts.
All
Company products are tested in certified labs in accordance with
MOH standards and are certified before being packaged and labelled
with detailed information regarding the THC, CBD and CBN content of
each product.2
_____________
1 According to a survey carried out by Cannabis
Magazine among 519 patients licensed by the MOH to consume medical
cannabis (Aug 2020, Israel).
2 The actual percentages of THC and CBD content are
determined by certified laboratory inspections and disclosed on the
label of each IMC-branded medical cannabis product sold in Israel.
Depending on such THC and CBD content, each IMC-branded medical
cannabis product is labelled based on the following categories, in
accordance with MOH regulations: (a) ‘T20/C4’ (THC
16-24% and CBD 0-7%); (b) ‘T15/C3’ (THC 11-19% and CBD
0-5.5%); (c) ‘T10/C2’ (THC 6-14% and CBD 0-3.8%); (d)
‘T10/C10’ (THC 6-14% and CBD 6-14%); (e)
‘T5/C5’ (THC 1-9% and CBD 1-9%); (f)
‘T0/C24’ (THC 0-0.5% and CBD 20-28%); (g)
‘T1/C20’ (THC 0-2.5% and CBD 16-24%); (h)
‘T3/C15’ (THC 0.5-5.5% and CBD 11-19%); and (i)
‘T5/C10’ (THC 2.5-7.5% and CBD 6-14%). The stated THC
and CBD percentage ranges for the IMC branded strains are expected
ranges; the actual percentages, as labelled on product packaging
under the IMC brand may vary or deviate from such ranges. The CBN
content is lower than 1.5% in all products sold in
Israel.
IM
Cannabis Corp.
Management’s Discussion and Analysis (Canadian dollars, in
thousands)
The
following graphic highlights key products sold under the IMC brand
in Israel:
Europe
In
Germany, the Company sells IMC-branded dried flower products. The
medical cannabis products sold in the German market are branded
generically as “IMC” so as to rely on the
Company’s brand recognition in establishing a foothold with
German healthcare professionals.
Canada
In
Canada, the Company’s product portfolio consists of dried
flowers, pre-rolled cannabis, pressed hash, and kief offerings sold
by TJAC under the WAGNERS brand into the Canadian adult-use
recreational cannabis market (rebranded from ‘JWC’ to
‘WAGNERS’ in May 2021). The WAGNERS brand competes in
the premium segment of the market. Dried flower is sold primarily
in 3.5 gram, 7 gram and 28 gram formats, pre-rolls are sold in a 3
x 0.5 gram format, and both hash and kief are sold in 1, 2 and 4
gram formats. Historically, TJAC sold cannabis on a
business-to-business basis to other Canadian licensed cannabis
producers. Recently, TJAC has shifted its focus to higher margin
business-to-consumer sales. With the acquisition of MYM, TJAC
expects to optimize the cultivation and brand footprint of both
WAGNERS and Highland Grow. IMCC intends to export premium cannabis
strains from TJAC and MYM as well as selected genetics from their
ultra-premium strains to the Israeli market for further fulfillment
of its global cultivation and distribution platform.
IM
Cannabis Corp.
Management’s Discussion and Analysis (Canadian dollars, in
thousands)
The
following graphic highlights key products sold under the WAGNERS
brand:
On July
9, 2021, the Company closed the MYM Transaction. Through this
acquisition, the Company acquired the rights to the Highland Grow
brand, a well-known, widely-available ultra-premium cannabis brand
in Canada. Highland Grow products include dried flower and
pre-rolled cannabis, with dried flower potency typically above 25%
THC. In addition to the proven track record of the Highland Grow
brand, MYM holds over 150 strains in its product portfolio that the
Company plans to selectively release to market.
IM
Cannabis Corp.
Management’s Discussion and Analysis (Canadian dollars, in
thousands)
The
following graphic highlights key products sold under the Highland
Grow brand:
Corporate
Developments
(i) the
Reverse Takeover Transaction and Liquidity Events
On
October 11, 2019, the Company completed a business combination with
IMC Holdings resulting in a reverse takeover of the Company by
shareholders of IMC Holdings (the “Reverse Takeover
Transaction”). The Reverse Takeover Transaction was effected
by way of a “triangular merger” between the Company,
IMC Holdings and a wholly-owned subsidiary of the Company pursuant
to Israeli statutory law.
In
connection with the Reverse Takeover Transaction, the Company
completed a private placement offering of 19,460,527 (on a
pre-Share Consolidation (as defined below) basis) subscription
receipts (each a “Subscription Receipt”) of a
wholly-owned subsidiary of the Company (“Finco”) at a
price of $1.05 per Subscription Receipt for aggregate gross
proceeds of $20,433 (the “Financing”). Upon the
satisfaction or waiver of, among other things, all of the condition
precedents to the completion of the Reverse Takeover Transaction,
each Subscription Receipt was exchanged for one unit of Finco (a
“Finco Unit”) with each Finco Unit being comprised of
one (1) common share of Finco (a “Finco Share”) and
one-half of one (1/2) Finco Share purchase warrant (each whole
warrant, a “Finco Warrant”). Each whole Finco Warrant
was exercisable for one Finco Share at an exercise price of $1.30
until October 11, 2021. Upon closing of the Reverse Takeover
Transaction, the Finco Shares and Finco Warrants were exchanged on
a 1:1 basis for Common Shares and Common Share purchase warrants
(“Listed Warrants”) on economically equivalent terms. A
total of 9,730,258 Listed Warrants were issued and listed for
trading on the CSE under the ticker “IMCC.WT”. The
Listed Warrants expired on October 11, 2021.
IM
Cannabis Corp.
Management’s Discussion and Analysis (Canadian dollars, in
thousands)
In
connection with the Financing, IMC Holdings granted to the agents
who acted on its behalf, options to acquire 1,199,326 compensation
units (the “2019 Compensation Units”) at an exercise
price of $1.05 per 2019 Compensation Unit. Upon completion of the
Reverse Takeover Transaction, the 2019 Compensation Units were
exchanged for compensation options of the Company (the “2019
Compensation Options”). Each 2019 Compensation Option
consists of one (1) Common Share and one-half of one (1/2) Common
Share purchase warrant (the “Unlisted Warrants” and
together with the Listed Warrants, the “Warrants”) with
each whole Unlisted Warrant exercisable for one (1) Common Share at
an exercise price of $1.30 until August 30, 2022, with such
Unlisted Warrants issued as a result of exercises of 2019
Compensation Options and not listed for trading on any
exchanges.
Upon
the completion of the Reverse Takeover Transaction, the former
holders of IMC Holdings held approximately 84.28% of the issued and
outstanding Common Shares and the previous holders of Subscription
Receipts held approximately 13.35% of the Common Shares, in each
case, on a non-diluted basis.
On
November 5, 2019, the Common Shares began trading on the CSE under
the ticker symbol “IMCC”.
On
February 12, 2021, the Company’s shareholders approved at a
special meeting the consolidation of all the Company’s issued
and outstanding Common Shares on a four (4) to one (1) basis (the
“Share Consolidation”). Following the Share
Consolidation, the number of Listed Warrants, Unlisted Warrants,
and 2019 Compensation Options outstanding was not altered; however,
the exercise terms were adjusted such that four Listed Warrants
were required to be exercised to purchase one Common Share
following at an adjusted exercise price of $5.20, four (4) Unlisted
Warrants are required to be exercised to purchase one (1) Common
Share at an adjusted exercise price of $5.20, and four 2019
Compensation Options are required to be exercised to purchase one
(1) unit at an adjusted exercise price of $4.20, with each unit
exercisable into one (1) Common Share and one-half of one (1/2)
Unlisted Warrant, with each whole Unlisted Warrant expiring on
August 30, 2022 and exercisable to purchase one (1) Common Share at
an exercise price of $5.20. The consolidated financial statements
give effect to the Share Consolidation for all periods
presented.
On
March 1, 2021, the Common Shares commenced trading on NASDAQ under
the ticker symbol “IMCC”, making the Company the first
Israeli medical cannabis operator to list its shares on
NASDAQ.
As of
September 30, 2021, and 2020, there were 7,362,762 and 9,729,735
Listed Warrants outstanding, respectively, re-measured by the
Company, according to their trading price in the market, in the
amount of $74 and $2,432, respectively. For the nine months ended
September 30, 2021 and 2020, the Company recognized a revaluation
gain (loss) of $15,856 and $(2,432), respectively. For the three
months ended September 30, 2021 and 2020, the Company recognized a
revaluation gain (loss) of $3,154 and $(973) in the consolidated
statement of profit or loss and other comprehensive income, in
which the unrealized gain is included in finance income
(expense).
As of
September 30, 2021, and 2020, there were 3,043,478 and nil Unlisted
Warrants outstanding, respectively, re-measured by the Company,
using the Black-Scholes pricing model, in the amount of $6,451 and
$nil, respectively. For the nine months ended September 30, 2021
and 2020, the Company recognized a revaluation gain (loss) of
$5,381 and $nil, respectively. For the three months ended September
30, 2021 and 2020, the Company recognized a revaluation gain (loss)
of $4,965 and $nil in the consolidated statement of profit or loss
and other comprehensive income, in which the unrealized gain is
included in finance income (expense).
IM
Cannabis Corp.
Management’s Discussion and Analysis (Canadian dollars, in
thousands)
During
the nine months ended September 30, 2021, a total of 2,366,496
Listed Warrants were exercised for 591,624 Common Shares at an
adjusted exercise price of $5.20 per Common Share. As a
result, the Company received a total amount of $3,075.
During
the nine months ended September 30, 2021, a total of 194,992
Unlisted Warrants were exercised for 48,748 Common Shares at an
adjusted exercise price of $5.20 per Common Share. As a
result, the Company received a total amount of $255.
During
the nine months ended September 30, 2021, a total of 197,632 2019
Compensation Options were exercised for 49,408 Common Shares and
24,703 Unlisted Warrants. Consequently, the Company received an
aggregate adjusted exercise amount of $208.
(ii) Restructuring
Current
Israeli law requires prior approval by the IMCA, a unit of the MOH,
of the identity of any shareholder owning 5% or more of an Israeli
company licensed by the IMCA to engage in cannabis-related
activities in Israel. For a number of reasons, including the
opportunity to leverage a network of multiple Israeli licensed
producers cultivating under the IMC brand, and in contemplation of
a “go-public transaction” to geographically diversify
the Company’s share ownership, IMC Holdings restructured its
organization on April 2, 2019 (the “IMC Restructuring”)
resulting in the divestiture to Oren Shuster and Rafael Gabay of
its interest in Focus, which is licensed by the IMCA to propagate
and cultivate medical cannabis in Israel.
IMC
Holdings retains an option with Messrs. Shuster and Gabay to
re-acquire the sold interest in Focus at its sole discretion and in
accordance with Israeli cannabis regulations, within 10 years of
the date of the IMC Restructuring (the “Focus
Agreement”). The Focus Agreement sets an aggregate exercise
price equal to NIS 765.67 per share of Focus for a total
consideration of NIS 2,756,500, that being equal to the price
paid by Messrs. Shuster and Gabay for the acquired interests in
Focus at the time of the IMC Restructuring.
As part
of the IMC Restructuring, IMC Holdings and Focus entered into an
agreement in which Focus shall use the IMC brand on an exclusive
basis for the sale of any cannabis plant and/or cannabis product
produced by Focus, either alone or together with other
sub-contractors engaged by Focus (the “IP Agreement”).
Focus is also obligated to exclusively use IMC Holdings for certain
management and consulting services including: (a) business
development services; (b) marketing services;
(c) strategic advisory services; (d) locating potential
collaborations on a worldwide basis; and (e) financial
analysis services (the “Services Agreement” and
collectively with the IP Agreement, the “Commercial
Agreements”).
Under
the IP Agreement, IMC Holdings charges Focus an amount equal to 25%
of its revenues on a quarterly basis, which shall not be changed
without the consent of IMC Holdings, as consideration for
Focus’ use of certain trademarks, know-how, technology and
maintenance services provided by IMC Holdings.
Under
the Services Agreement, IMC Holdings charges Focus an amount equal
to IMC Holdings’ cost of providing certain services to Focus
plus a 25% mark-up, which shall not be changed without the consent
of IMC Holdings, as consideration for the provision of such
services.
Subsequent
to the IMC Restructuring, according to accounting criteria in IFRS
10, the Company is still viewed as effectively exercising control
over Focus, and therefore, the accounts of Focus continue to be
consolidated with those of the Company.
IM
Cannabis Corp.
Management’s Discussion and Analysis (Canadian dollars, in
thousands)
As a
result of the IMC Restructuring, IMCC derives revenue from the
Commercial Agreements. IMCC does not directly hold any licenses to
engage in the cultivation, production, processing, distribution or
sale of medical cannabis in Israel.
(iii) Regulatory
Changes in Israel
Changes under the MOH Regulations
Until
September 2019, patients licensed for consumption of medical
cannabis products by the IMCA received all of their medical
cannabis products authorized under their respective licenses at a
fixed monthly price of NIS 370, regardless of each
patient’s authorized amount. As an example, a patient who was
to receive 20 grams of medical cannabis products per month would
pay the same monthly fee of NIS 370 as a patient who received
180 grams per month. In addition, IMCA assigned patients to a
particular licensed medical cannabis producer, from which each
patient would exclusively receive their medical cannabis products.
Under the previous medical cannabis regulations, Focus distributed
approximately 80% of its medical cannabis products via home
delivery and the remaining 20% via an IMCA-established distribution
outlet.
Under
the MOH’s new regulations, medical cannabis products are
delivered from a licensed producer to a manufacturer, which then
delivers to a distributor to distribute to pharmacies. In addition,
patients licensed for consumption of medical cannabis products are
no longer exclusively assigned to medical cannabis producers and
may purchase medical cannabis products from authorized pharmacies
at a range of price points without any MOH-regulated price
controls.
In
light of the MOH’s new regulations, some medical cannabis
patient licenses granted under the previous regime are still valid.
The medical cannabis patient licenses set to expire during the
period from February 1, 2019 to July 31, 2019 were extended by
order of the Israeli Supreme Court until further notice by the
Court. While these licenses remain valid, the patients who hold
these licenses are entitled to receive medical cannabis products
pursuant to the price controls and supplier restrictions of the
former regime. Additional information on the proceedings pursuant
to which the above-referenced order was granted can be found under
“Legal Proceedings and Regulatory Actions – Legal
Proceedings – Supreme Court of Justice
2335/19”.
Following
the implementation of the above MOH’s new regulations, the
Group believes that the Israeli medical cannabis market will
continue to benefit from price stability of the premium and super
premium medical cannabis products, an increase to the number of
physicians certified by the IMCA to prescribe medical cannabis and
thus, an increase in the number of licensed medical cannabis
patients.
Medical Cannabis Imports
In
October 2020, the IMCA issued an updated procedure, titled
“Guidelines for Approval of Applications for Importation of
Dangerous Drug of Cannabis Type for Medical Use and for
Research” (“Procedure 109”), describing the
application requirements for cannabis import licenses for medical
and research purposes. According to Procedure 109, the following
permits and licenses are required to receive a cannabis import
license: (1) License to possess medical cannabis and operate
in the medical cannabis industry; (2) License to import plant
material; (3) Permit to import narcotic drugs; and
(4) License to import a dangerous drug.
IM
Cannabis Corp.
Management’s Discussion and Analysis (Canadian dollars, in
thousands)
Medical Cannabis Exports
In
October 2020, the MOH launched a new pilot program under which
medical cannabis producers would be authorized to export medical
cannabis products, subject to the requirement that certain products
be made available at a fixed price of NIS 14 per gram to
patients in Israel over the age of 21 and NIS 10 per gram to
patients under the age of 21 (the “Pilot Program”).
Products bearing the IMC brand were offered as part of the Pilot
Program during the first and second quarter of 2021. The Pilot
Program expired at the end of Q1 2021 and was not extended by the
MOH.
In
December 2020, the IMCA published guidelines for the medical
cannabis export permit application process,3 pursuant to which an
export permit will only be granted to an applicant if
(i) sufficient domestic supply has been secured by such
applicant in the variety and quantity that will meet the Israeli
level of demand; (ii) the delivery of medical cannabis is made
from approved sites; (iii) the applicant has a valid IMC-GDP
certification and business license from the IMCA; and (iv) an
import permit from the importing country is obtained and attached
to the export application.
Legalization of Adult-Use Recreational Cannabis in
Israel
As of
the date of this MD&A, adult-use recreational cannabis use in
Israel is illegal. In November 2020, an Israeli government
committee responsible for advancing the cannabis market reform
published a report supporting and recommending the legalization of
adult-use recreational cannabis in Israel (the
“Report”). Based on the Report, the Israeli Ministry of
Justice was expected to formulate a bill to begin the legislative
process towards the legalization of adult-use recreational
cannabis. The government committee made its recommendation for
legalization based on the increasing demand for adult-use
recreational cannabis in Israel, the importance of maintaining
quality standards and limiting uncontrolled products, the need for
increased access to cannabis by medical patients and the objective
of decreasing the size of the illegal market. The model proposed by
the government committee in the Report is similar in nature to the
model adopted in Canada, whereby the sale of adult-use recreational
cannabis would be channeled through government-licensed
dispensaries.
In
December 2020, the governing Israeli parliament dissolved and the
then-existing draft bill regarding the proposed legalization of
adult-use recreational cannabis became defunct. However, the new
government, formed on June 13, 2021, declared and settled in the
coalition agreement, its commitment to legalization of adult-use
recreational cannabis. Since the formation of the new government,
several legislative initiatives were filed, including for the
decriminalization of the possession of cannabis for individual
recreational adult-use and the legalization of CBD for non-medical
use. These initiatives were not accepted, however they are viewed
as first steps towards more comprehensive legislation towards the
legalization of adult-use recreational cannabis. Members of the
Israeli government continue to affirm their commitment to the
legalization of adult-use recreational cannabis.
(iv) Israeli
Market
The
Israeli medical cannabis market has shown dramatic growth over the
past several years. It is projected that this growth will continue
and according to MOH estimates, the number of patients in Israel
licensed by the MOH to consume medical cannabis is expected to
reach approximately 120,000 by the end of 2021.
IM
Cannabis Corp.
Management’s Discussion and Analysis (Canadian dollars, in
thousands)
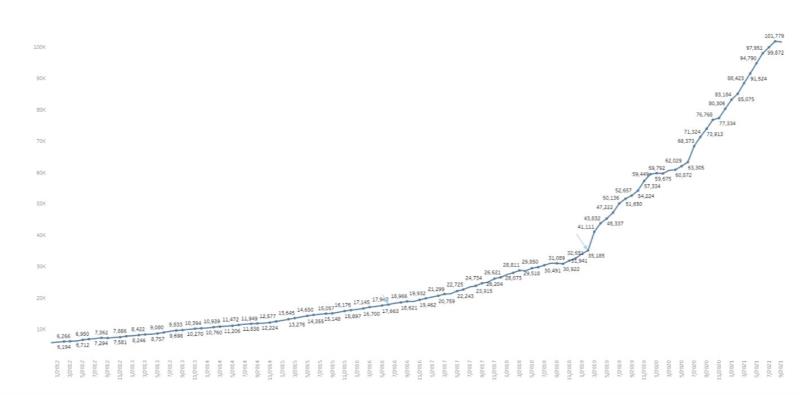
Israeli Market Development 2011-2021
According
to MOH monthly publication, as of September 2021, there are 101,779
licensed patients in Israel, and a monthly prescription of
3,861,000 and 2,848,000 grams of cannabis were recorded in
September 2021 and December 2020, respectively.3
The
below reflects the number of licensed medical cannabis patients in
Israel over the year 2011 to September 2021:4
(v) Canadian
Market
On
October 17, 2018, the Cannabis
Act came into effect in Canada, regulating both medical and
recreational cannabis. Under this legislation, cannabis cultivators
and processors are licenced by Health Canada to cultivate, produce
or sell cannabis products for medical and recreational consumption.
Retail activities in the Canadian cannabis industry are licensed by
the relevant provincial or territorial government. Consumers do not
require a licence to purchase cannabis. In 2020, roughly 27% of
Canadians surveyed by Statistics Canada had consumed cannabis in
the past 12 months.5 From October 2018 to
August 2021, sales of legal recreational cannabis increased by
roughly 750%, to nearly $360,000,000 per month, and 20% from March
2021 to August 2021 alone.6 While the Canadian
market remains in its infancy, growth has been significant, due
partially to the increasing availability of retail cannabis stores.
As of October 2021, there were an estimated 2,500 cannabis stores
in Canada.7
________________
4 Ministry of Health – licensed
patients’ data as of September 2021 - https://www.health.gov.il/Subjects/cannabis/Documents/licenses-status-September-2021.pdf
5 Canadian Cannabis Survey 2020:
Summary. https://www.canada.ca/en/health-canada/services/drugs-medication/cannabis/research-data/canadian-cannabis-survey-2020-summary.html#a2
6 Retail trade sales by province and
territory. https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=2010000801&pickMembers%5B0%5D=2.30&pickMembers%5B1%5D=3.1&cubeTimeFrame.startMonth=10&cubeTimeFrame.startYear=2018&cubeTimeFrame.endMonth=12&cubeTimeFrame.endYear=2021&referencePeriods=20181001%2C20211201
7 Looking back from 2020, how
cannabis use and related behaviours changed in Canada.
https://www.cannabisbenchmarks.com/report-category/Canada/
IM
Cannabis Corp.
Management’s Discussion and Analysis (Canadian dollars, in
thousands)
(vi) European
Activity and the German Market
The
Company’s European strategy is centered in Germany, whose
medical cannabis market is currently considered the largest in
Europe.8
To develop its operations in Germany, on March 15, 2019, IMC
Holdings acquired 100% of the outstanding shares of Adjupharm (the
“Adjupharm Shares”), a licensed EU-GMP certified
medical cannabis producer and distributor, for approximately $1,400
(€924 as of the acquisition date) with additional obligations
to the sellers including repayment of bank loans of up to $1,030
(€680 as of the acquisition date). These bank loans were
repaid by IMC Holdings in May 2019. The Company, through IMC
Holdings, currently owns 90.02% of Adjupharm, with the balance
owned by Adjupharm’s Chief Executive Officer.
The
Company continues to develop Adjupharm as its European hub and to
expand its presence in the German market by forging partnerships
with pharmacies and distributors across the country. The
Company’s objective is to capture a significant market share
in Germany by working directly with pharmacies and with
distributors to increase market reach for products bearing the IMC
brand. The Company currently has approximately 8,000 square feet of
warehousing and GMP Standard production capacity in Germany,
following the recent completion of an expansion process of its
facility to a new, state-of-the-art logistics centre, upgrading its
production technology and increasing its storage capacity to seven
tons of cannabis.
Adjupharm
sources its supply of medical cannabis for the German market from
various EU-GMP standard European and Canadian suppliers, and is
actively seeking additional supply partners to diversify its source
of supply of premium and super premium cannabis products and
minimize the risks inherent in the supply chain.
Adjupharm
relies on its sales and distribution agreements to supply and
distribute IMC-branded products to distribution partners or
directly to German pharmacies. There are approximately 19,000
community pharmacies in Germany, each of which is permitted to
create and dispense medications, including medical cannabis,
pursuant to physician prescriptions.9 In the first quarter
of 2021, Adjupharm completed the expansion of its internal and
external sales department and is focused on increasing physician
awareness and engagement to drive sales of IMC-branded medical
cannabis products. In July 2021, Adjupharm was recognized by the
German Brand Institute with the “German Brand Award
2021”, recognizing its excellence in brand strategy and
creation, communication and integrated marketing. The competitive
advantage in Germany also lies in the Group’s track record
and brand reputation in Israel and proprietary data supporting the
effectiveness of medical cannabis for the treatment of a variety of
conditions.
The
Company has also engaged in exploratory operations to expand to
Portugal and Greece, by establishing a wholly-owned subsidiary in
Portugal in October 2018, and a joint venture in Greece (25% owned
by IMCC), however it has deferred any further investment in these
jurisdictions indefinitely in light of the uncertainty related to
COVID-19.
_______________
8 Health Europa, June 23, 2020. https://www.healtheuropa.eu/exploring-growth-in-the-european-medical-cannabis-market/100849/
IM
Cannabis Corp.
Management’s Discussion and Analysis (Canadian dollars, in
thousands)
Due to
the impact of the COVID-19 pandemic on Germany in the first quarter
of 2021, the Company, through Adjupharm, leveraged its established
distribution platform to enter into several reseller agreements of
COVID-19 antigen test kits. Such engagement of Adjupharm facilitate
and further enhance its business relationship with pharmacies in
Germany and support its distribution platform for medical cannabis.
Due to the evolving impact of COVID-19 worldwide, and in light of
the uncertainty related to the demand for COVID-19 test kits in
Germany, the Company is examining the potential demand for such
test kits in Israel. For more information, please see “Strategic
Developments”.
(vii) Investment
in Xinteza
On
December 26, 2019, IMC Holdings entered into a share purchase
agreement with Xinteza, a company with a unique biosynthesis
technology, whereby the Company acquired, on an as-converted and
fully diluted basis, 25.37% of Xinteza’s outstanding share
capital, for consideration of US$1,700 (approximately $2,300 as of
September 30, 2021) paid in several installments (the
“Xinteza SPA”). As of September 30, 2021, the Company
has paid all outstanding installments pertaining to the Xinteza SPA
and currently holds 23.35% of the outstanding share capital of
Xinteza on an as-converted and fully diluted basis.
Under
an exclusive license from Yeda Research & Development Company
Ltd., the commercial division of the Weizmann Institute of Science,
and based on disruptive plant genetics and metabolomics research
led by Professor Asaph Aharoni, Xinteza has been developing
advanced proprietary technologies relating to the production of
cannabinoid-based active pharmaceutical ingredients for the
pharmaceutical and food industries using biosynthesis and
bio-extraction technologies.
(viii) Strategic
Developments:
1.
On July 9, 2021,
the Company closed the MYM Transaction, implemented in accordance
with the terms and conditions of the arrangement agreement dated
March 31, 2021, between IMCC, MYM and Trichome, which resulted in
the acquisition by IMCC of all of the issued and outstanding shares
of MYM (the “MYM Shares”) in exchange for 0.022 of a
Common Share for each MYM Share. In connection with the MYM
Transaction, a total of 10,073,437 Common Shares have been issued
to the former holders of MYM Shares, resulting in former MYM
shareholders holding approximately 15% of the total number of
Common Shares at the time of issuance (based on 67,156,470 Common
Shares issued and outstanding immediately after
closing).
2.
On July 28, 2021,
IMC Holdings entered into a definitive agreement in respect of the
Pharm Yarok Transaction. The aggregate consideration for the Pharm
Yarok Transaction is NIS 11,900 (approximately $4,600), of which
NIS 3,500 (approximately $1,300) shall be invested in the Company
at closing by the shareholders of Pharm Yarok Group in exchange for
Common Shares. Closing of the Pharm Yarok Transaction is
conditional upon receipt of all requisite approvals, including from
the MOH. Pharm Yarok is a leading medical cannabis pharmacy and
trade company located in central Israel; Rosen High Way is a trade
and distribution centre providing medical cannabis storage,
distribution services and logistics solutions for cannabis
companies and pharmacies in Israel; and HW Shinua is an applicant
for a medical cannabis transportation license from the IMCA, the
receipt of which would permit HW Shinua to transport large
quantities of medical cannabis to and from Pharm Yarok’s
pharmacy and Rosen High Way’s distribution centre and to and
from third parties in the medical cannabis sector, including
medical cannabis growing facilities, pharmacies, manufacturers and
distribution centres across Israel.
IM
Cannabis Corp.
Management’s Discussion and Analysis (Canadian dollars, in
thousands)
3.
On July 30, 2021,
in connection with the Panaxia Transaction, the Company issued the
first installment of 142,007 Panaxia Consideration Shares at a
price of US$5.009 (approximately $6.24) per Panaxia Consideration
Share, representing an aggregate value equal to approximately
US$711 (approximately $889), with up to four additional
installments to follow. The issue price of the Panaxia
Consideration Shares was calculated based on the average closing
price of the Common Shares on NASDAQ over the 10 trading day period
immediately preceding July 30, 2021.
4.
On August 3, 2021,
IMC Holdings and cbdMD executed a binding letter of intent that
will grant IMC Holdings an exclusive right to import, sell,
distribute and market cbdMD products in Israel using the cbdMD
brand name and trademark, subject to the legalization of
hemp-derived CBD for non-medical purposes in Israel.
5.
On August 16, 2021,
IMC Holdings entered into definitive agreement with Vironna in
connection with the Vironna Transaction to acquire 51% of the
issued and outstanding ordinary shares of Vironna for aggregate
consideration of NIS 8,500 (approximately $3,300), comprised of NIS
5,000 (approximately $1,950) in cash and NIS 3,500 (approximately
$1,350) in Common Shares to be issued at closing date (the
“Vironna Share Consideration”). Closing of the Vironna
Transaction is conditional upon receipt of all requisite approvals,
including from the MOH. To satisfy the Vironna Share Consideration,
the Company will issue number of Common Shares calculated based on
the average closing price of the Common Share on NASDAQ over the 14
trading day period immediately preceding the date of issuance.
Vironna is a leading pharmacy licensed to dispense and sell medical
cannabis to licensed medical cannabis patients, located in central
Israel and is one of the leading pharmacies serving patients among
the Arab population in Israel.
6.
On August 19, 2021,
Focus Medical Herbs Ltd., issued its first purchase order for
approximately 220 kilograms of medical cannabis purchased from The
Flowr Corporation (“Flowr”), under a three-year supply
agreement entered into by Focus and Flowr on June 24, 2021. This
order is expected to be exported to Israel in Q4 2021, subject to
the satisfaction of applicable regulatory and import requirements.
Flowr is a Canadian licensed producer of ultra-premium adult-use
recreational and medical cannabis products.
7.
On September 1,
2021, the Issuer issued the second instalment of 246,007 Panaxia
Consideration Shares at a price of US$3.68 (approximately $4.64)
per Panaxia Consideration Share, representing an aggregate value of
US$905 (approximately $1,141). The issue price of the Panaxia
Consideration Shares was calculated based on the average closing
price of the Common Shares on NASDAQ over the 10 trading day period
immediately preceding September 1, 2021.
8.
On September 17,
2021, Adjupharm signed a five-year exclusive supply agreement for
the German market with Zelira Therapeutics Ltd.
(“Zelira”). Pursuant to the agreement, Adjupharm will
distribute Zelira’s Zenivol product in Germany. Zenivol is a
proprietary cannabinoid-based medicinal product for treatment of
chronic insomnia, supported by clinical data.
IM
Cannabis Corp.
Management’s Discussion and Analysis (Canadian dollars, in
thousands)
9.
On September 23,
2021, TJAC increased the limit on the revolving credit facility
entered into with a private Canadian creditor on May 17, 2021 (the
“Facility”) from $5,000 to $7,500. The increase will be
used to finance TJAC’s receivables in order to manage the
timing of its cash flows. The revised commitment from the private
Canadian creditor is $7,500, with an initial term of 12 months that
can be extended upon the mutual agreement of both parties. Per
annum interest is equal to the greater of (i) 9.75% and, (ii) the
Toronto Dominion Bank prime rate, plus 7.30%. The Facility has a
standby fee of 2.40% per annum, which is charged against the unused
portion. Advanced amounts are secured against the assets of TJAC
and Trichome, with Trichome providing a guarantee for the
Facility. To maintain
the Facility, TJAC must abide by certain financial covenants, such
as the maintenance of a tangible net worth greater than $5 million
and a debt service coverage ratio of 2:1.
Subsequent Events
1.
On October 15,
2021, the Issuer issued the third instalment of 248,212 Panaxia
Consideration Shares at a price of US$3.225 (approximately $4.01)
per Panaxia Consideration Share, representing an aggregate value
equal to US$800 (approximately $996). The issue price of the
Panaxia Consideration Shares was calculated based on the average
closing price of the Common Shares on NASDAQ over the 10-trading
day period immediately preceding October 1, 2021.
Company Outlook
Israel
In
Israel, the Company, through the Commercial Agreements, continues
to expand the IMC brand recognition, and, in association with
Focus, supply the growing Israeli medical cannabis market with
products bearing the IMC brand. With the expected high growth of
the Israeli medical cannabis market, the Company is well positioned
to benefit from its long-term presence and strong brand
recognition, expecting a continued increase in revenues and
profitability. In addition, the Company intends to enter, through
its subsidiaries, additional segments of the medical cannabis
market in Israel, including the distribution and retail segments,
by completing the Panaxia Transaction, the Pharm Yarok Transaction,
and the Vironna Transaction. Following the completion of the
foregoing transactions, the Company expects vertical integration to
increase its revenue and margins from its Israeli medical cannabis
market activities, diversify its business opportunities and gain
direct access to medical cannabis patients to benefit from market
knowledge and trends. Furthermore, the Group is focused on
diversifying its product portfolio with premium and super premium
medical cannabis products, leveraging its Canadian cultivation
facilities that are expected to provide additional opportunities to
export premium cannabis products to Israel and
Germany.
Europe
The
Company’s objective within Europe is to capitalize on the
increasing demand for medical cannabis products and to bring the
well-established IMC brand and its product portfolio to European
patients. The Company’s operating track record, accumulation
of data and brand reputation in Israel is a competitive advantage
in gaining traction within the German and European markets and
building support among physicians who prescribe medical cannabis
products.
IM
Cannabis Corp.
Management’s Discussion and Analysis (Canadian dollars, in
thousands)
Canada
Following
the successful completion of the acquisition of Trichome on March
18, 2021 (the “Trichome Transaction”) and the MYM
Transaction on July 9, 2021, IMCC plans to integrate and manage its
assets in Canada with a goal of maximizing Company-wide revenue and
margins.
Additionally,
the Company will continue to drive organic growth from Canadian
operations through active portfolio management of its products,
additional SKU launches, boosting cultivation efficiency, and
adding to the number of points of distribution across the country.
The Company expects to continue to drive meaningful revenue growth
in the near-term by diversifying its product portfolio under the
WAGNERS and Highland Grow brands, including the introduction of new
dried flower, pre-rolled, and hash SKUs in key Canadian
markets.
Overview of Financial Performance
|
|
For the nine
months ended
September
30,
|
For the three months ended
September 30,
|
For the Year ended December 31,
|
|
|
|
|
|
|
|
|
Net
Revenues
|
$34,272
|
$10,990
|
$14,393
|
$5,893
|
$15,890
|
|
Gross profit before
fair value impacts in cost of sales
|
$8,109
|
$6,018
|
$2,880
|
$3,362
|
$8,809
|
|
Gross margin before
fair value impacts in cost of sales (%)
|
24%
|
55%
|
20%
|
57%
|
55%
|
|
Operating
Loss
|
$(26,667)
|
$(1,862)
|
$(14,245)
|
$(671)
|
$(8,245)
|
|
Loss
|
$(6,030)
|
$(8,758)
|
$(5,656)
|
$738
|
$(28,734)
|
|
Loss per share
attributable to equity holders of the Company - Basic
|
$(0.10)
|
$(0.06 (
|
$(0.06 (
|
$0.004
|
$(0.74)
|
|
Loss per share
attributable to equity holders of the Company - Diluted (in
CAD)
|
$(0.51)
|
$(0.06 (
|
$(0.18 (
|
$0.004
|
$(0.74)
|
The
Overview of Financial Performance includes reference to
“gross margin”, which is a non-IFRS financial measure.
Non-IFRS measures are not recognized measures under IFRS, do not
have a standardized meaning prescribed by IFRS and are therefore
unlikely to be comparable to similar measures presented by other
companies. The Company defines gross margin as the difference
between revenue and cost of revenues divided by revenue (expressed
as a percentage), prior to the effect of a fair value adjustment
for inventory and biological assets.
IM
Cannabis Corp.
Management’s Discussion and Analysis (Canadian dollars, in
thousands)
Operational Results - Medical Cannabis
|
|
For the Nine
Months Ended
September
30,
|
For the Three months ended
September 30,
|
For the Year ended December 31,
|
|
|
|
|
|
|
|
|
Average net selling
price of dried flower (per Gram)
|
$4.56
|
$5.50
|
$4.85
|
$5.49
|
$5.75
|
|
Average net selling
price of other cannabis products (per Gram)1
|
$5.30
|
-
|
$6.31
|
-
|
-
|
|
Quantity harvested
and trimmed (in Kilograms)2
|
2,772
|
2,954
|
1,490
|
-
|
2,545
|
|
Quantity of other
cannabis products sold (in Kilograms)1
|
530
|
-
|
327
|
-
|
-
|
|
Quantity of dried
flower sold (in Kilograms)
|
5,461
|
1,506
|
2,434
|
981
|
2,586
|
Notes:
(1)
Including other
cannabis products such as Kief, Hash and Pre-rolls.
(2)
Harvested flowers,
after trimming and ready for manufacturing.
Review of Operations for the Nine and Three Months Ended September
30, 2021 and 2020
Net Revenues
The
Group operates in one reporting segment. The main revenues of the
Group are generated from sales of medical cannabis products to
customers in Israel and Germany as well as products to the
recreational market in Canada.
Revenues
for the nine months ended September 30, 2021 and 2020 were $34,272
and $10,990, respectively, representing an increase of $23,282 or
212%. Revenues for the three months ended September 30, 2021 and
2020 were $14,393 and $5,893, respectively, representing an
increase of $8,500 or 144%. Total dried flower sold for the nine
months ended September 30, 2021 was 5,461kg at an average selling
price of $4.56 per gram compared to 1,506kg for the nine months
ended September 30, 2020 at an average selling price of $5.50 per
gram, derived mainly from the lower average selling price per gram
the Company adopted with its new acquisitions in the Canadian
market. Total dried flower sold for the three months ended
September 30, 2021 was 2,434kg at an average selling price of $4.85
per gram compared to 981kg for the three months ended September 30,
2020 at an average selling price of $5.49 per gram. The increase in
revenues related to dried flower for the nine and three months
ended September 30, 2021 is attributable to deliveries made under
the Focus’ sales agreements to pharmacies, as well as to
revenues generated from Adjupharm, consolidation of Trichome, MYM,
and the Consolidated Entities according to the definitive agreement
dates for each of the Consolidated Entities. Total other cannabis
product sold for the nine months ended September 30, 2021 was 530kg
at an average selling price of $5.3 per gram compared to nil for
the nine months ended September 30, 2020 at an average selling
price of $nil per gram. Total other cannabis product sold for the
three months ended September 30, 2021 was 327kg at an average
selling price of $6.31 per gram compared to nil for the three
months ended September 30, 2020 at an average selling price of $nil
per gram. The increase in revenues related to other cannabis
products for the nine and three month ended September 30, 2021 is
attributable to TJAC brands after the consolidation of Trichome
activities.
IM
Cannabis Corp.
Management’s Discussion and Analysis (Canadian dollars, in
thousands)
Cost of Revenues
The
cost of revenues includes the purchase of raw materials,
production, product testing, shipping and sales related costs. At
harvest, the biological assets are transferred to inventory at
their fair value which becomes the deemed cost for the inventory.
Inventory is later expensed to the cost of sales when sold. Direct
production costs are expensed through the cost of
sales.
The
cost of revenues for the nine months ended September 30, 2021 and
2020 were $26,163 and $4,972, respectively, representing an
increase of $21,191 or 426%. Cost of revenues for the three months
ended September 30, 2021 and 2020 were $11,513 and $2,531,
respectively, representing an increase of $8,982 or 355%. Cost of
revenues is comprised of cultivation costs, purchase of materials
and finished goods, utilities, salary expenses and import costs.
Focus, Highland and TJAC expect net cost of sales to vary from
quarter to quarter based on the number of pre-harvest plants, after
harvest plants, the strains being grown and technological progress
in the trimming machines.
Gross Profit
Included
in the Company’s calculation of gross profit are the
following:
●
production costs
(current period costs that are directly attributable to the
cannabis growing and harvesting process);
●
materials and
finished goods purchase costs
●
a fair value
adjustment on sale of inventory (the change in fair value
associated with biological assets that were transferred to
inventory upon harvest);
●
a fair value
adjustment on growth of biological assets (the estimated fair value
less cost to sell of biological assets as at the reporting
date).
Included
in gross profit is the net change in fair value of biological
assets, inventory expensed and production costs. Biological assets
consist of cannabis plants at various after-harvest stages which
are recorded at fair value less costs to sell after
harvest.
Gross
profit for the nine months ended September 30, 2021 and 2020 was
$5,985 and $9,961, respectively, representing a decrease of $3,976
or 40%. For the three months ended September 30, 2021 and 2020
gross profit was $1,525 and $3,381, respectively, representing a
decrease of $1,856 or 55%. Gross profit included gains (losses)
from unrealized changes in fair value of biological assets and
realized fair value adjustments on inventory sold of $(2,124) and
$3,943 for the nine months ended September 30, 2021 and 2020,
respectively. Gains (losses) from unrealized changes in fair value
of biological assets and realized fair value adjustments on
inventory sold for the three months ended September 30, 2021 and
2020 were $(1,355) and $19, respectively. Gross profit was impacted
primarily due to a delay in a certain shipment in TJAC that was
scheduled to occur prior to quarter end but occurred at the
beginning of October 2021. Given the largely fixed cost structure
at TJAC, gross margins are sensitive to changes in revenue and are
expected to increase with revenue growth.
IM
Cannabis Corp.
Management’s Discussion and Analysis (Canadian dollars, in
thousands)
Expenses
General and Administrative
General
and administrative expenses for the nine months ended September 30,
2021 and 2020 were $22,634 and $7,223, respectively, representing
an increase of $15,411 or 213%. For the three months ended
September 30, 2021 and 2020, general and administrative expenses
were $10,246 and $2,197, respectively, representing an increase of
$8,049 or 366%. The increase in the general and administrative is
mainly attributable to the growing corporate activities in Israel,
Canada, and Germany, professional services derived from legal fees
and other consulting services, among other, in relation to the
NASDAQ listing and M&A processes in the amount of $8,838
(including share-based expenses to financial advisors of
approximately $1,301), salaries to employees in the amount of
$6,426, impairment of other receivables in the amount of $570 and
insurance costs in the amount of $2,002.
Selling and Marketing
Selling
and marketing expenses for the nine months ended September 30, 2021
and 2020 were $4,654 and $2,334, respectively, representing an
increase of $2,320 or 99%. For the three months ended September 30,
2021, selling and marketing expenses were $2,169, compared to
$1,150 for the three months ended September 30, 2020, representing
an increase of $1,019 or 89%. The increase in the selling and
marketing expenses was due mainly to the Company’s increased
marketing efforts in Israel and brand launch in Germany, as well as
increased distribution expenses relating to the increase in sales,
full quarter consolidation of Trichome’s results and nearly a
full quarter of consolidation of MYM’s results.
Research and Development
Research
and development expenses for the nine months ended September 30,
2021 and 2020 were $10 and $135, respectively, representing a
decrease of $125 or 93%. For the three months ended September 30,
2021 and 2020, research and development expenses were $4 and $1,
respectively, representing an increase of $3 or 300%. The decrease
for the nine and three months ended September 30, 2021 was
primarily associated with the COVID-19 pandemic, which caused
delays in new projects.
Share-Based Compensation
Share-based
compensation expense for the nine months ended September 30, 2021
and 2020 was $5,354 and $2,131, respectively, representing an
increase $3,223 or 151%. For the three months ended September 30,
2021 and 2020, share-based compensation expense was $3,351 and
$704, respectively, representing an increase of $2,647 or 376%. The
increase was mainly due to the grant of new incentive stock options
(“Options”).
Financing
Financing
income (expense), net, for the nine months ended September 30, 2021
and 2020 was $20,812 and $(5,975), respectively, representing an
increase of $26,787 or 448%. For the three months ended September
30, 2021 and 2020, financing income was $8,224 and $1,186,
respectively, representing an increase of $7,038 or 593%. The
change was mainly due to $21,169 finance income arising from
valuation update of the Warrants and other financial instruments,
which was affected by the Company’s decreased share
price.
IM
Cannabis Corp.
Management’s Discussion and Analysis (Canadian dollars, in
thousands)
Depreciation and Amortization
Depreciation
and amortization expenses for nine months ended September 30, 2021,
and 2020 were $3,604 and $672, respectively, representing an
increase of $2,932 or 436%. For the three months ended September
30, 2021 and 2020, Depreciation and amortization expenses were
$1,961 and $244, respectively, representing an increase of $1,717
or 704%. Depreciation and amortization expenses are impacted by the
adoption of IFRS 16 Leases, depreciation of PP&E, as well as
the amortization of intangible assets mainly following the
acquisitions of Trichome and MYM this year.
Net Income loss
Net
loss for the nine months ended September 30, 2021 and 2020 was
$(6,030) and $(8,758), respectively, representing a net loss
decrease of $2,728 or 31%. For the three months ended September 30,
2021 and 2020, Net income (loss) was $(5,656) and $738
respectively, representing a net loss increase of $6,394 or 866%.
The net income decrease related to factors impacting net income
from operations described above, and finance income driven by
revaluation of Warrants and other financial instruments in the
amount of $21,169, which were recorded against liability on the
grant day and were re-evaluated at September 30, 2021 through
profit or loss.
Net Income (Loss) per share Basic and Diluted
Basic
loss per share is calculated by dividing the net profit
attributable to common shareholders of the Company by the weighted
average number of Common Shares outstanding during the period.
Diluted profit per Common Share is calculated by adjusting the
earnings and number of Common Shares for the effects of dilutive
Warrants and other potentially dilutive securities. The weighted
average number of Common Shares used as the denominator in
calculating diluted profit per Common Share excludes unissued
Common Shares related to Options as they are antidilutive. Basic
Income (Loss) per Common Share for the nine months ended September
30, 2021 and 2020 were $(0.10) and $(0.06) per Common Share,
respectively. For the three months ended September 30, 2021 and
2020 were $(0.06) and $0.004 respectively.
Diluted
Income (Loss) per Common Share for the nine months ended September
30, 2021 and 2020 were $(0.50) and $(0.06) per Common Share,
respectively. Diluted Income (Loss) per Common Share for the three
months ended September 30, 2021 and 2020 were $(0.18) and $0.004,
respectively.
Total Assets
Total
assets as at September 30, 2021 were $275,387, compared to $38,116
as at December 31, 2020, representing an increase of $237,271 or
622%. This increase was primarily due to the consolidation of
Trichome, MYM, and the Consolidated Entities, leading to
recognition of goodwill and intangible assets of an aggregate
amount of approximately $121,143, property plant and equipment of
approximately $22,741, increase in right-of-use assets of
approximately $15,667 and approximately $12,932 of working capital.
Additional increase of $39,622 in cash derived from the
Company’s financing round at May 2021.
IM
Cannabis Corp.
Management’s Discussion and Analysis (Canadian dollars, in
thousands)
Total Liabilities
Total
liabilities as at September 30, 2021 were $64,408, compared to
$25,506 at December 31, 2020, representing an increase of $38,902
or 153%. The increase was primarily due to an increase of $7,577 in
other accounts payable and accrued expenses, an increase of $5,594
in purchase consideration payable and an increase of $17,176 in
lease liabilities.
Intangible Assets
1.
On March 15, 2019,
IMC Holdings acquired Adjupharm, a licensed EU-GMP producer with
wholesale, narcotics handling and import/export licenses for
medical cannabis. As part of its global expansion and penetration
plan into the European market, IMCC acquired 100% of
Adjupharm’s issued and outstanding shares for €924
(approximately $1,400).
Through
the acquisition of Adjupharm, the Company recognized $1,287 in
intangible assets and goodwill. The goodwill arising on the
acquisition was attributed to the expected benefits from the
synergies of the combination of the activities of the Company and
Adjupharm.
The
goodwill recognized is not expected to be deductible for income tax
purposes.
The
Company recognized and updated the fair value of the assets
acquired and liabilities assumed in the business combination
according to a final valuation made by an external valuation
specialist.
2.
On March 18, 2021,
the Trichome Transaction was completed pursuant to the terms and
subject to the conditions of arrangement agreement dated December
30, 2020, whereby the Company agreed to acquire all the issued and
outstanding securities of Trichome pursuant to a statutory plan of
arrangement under the Business
Corporations Act (Ontario). As a result of the Trichome
Transaction, the businesses of IMCC and Trichome have been
combined. Upon completion of the Trichome Transaction, the total
Common Share consideration valued at approximately
$99,028.
Through
the acquisitions of Trichome, the Company recognized goodwill of
approximately $66,769 and intangible assets, primarily the
cultivation license, worth approximately $6,458 (based on a
preliminary purchase price allocation study). The goodwill arising
on acquisition is attributed to the expected benefits from the
synergies of the combination of the activities of the Company and
Trichome, as well as value attributed to the assembled workforce,
which is included in goodwill. The goodwill recognized is not
expected to be deductible for income tax purposes.
The
Company recognized the fair value of the assets acquired and
liabilities assumed in the business combination according to a
provisional measurement. As of the date of the approval of the
Interim Financial Statements, a final valuation for the fair value
of the identifiable assets acquired and liabilities assumed by an
external valuation specialist has not been obtained. The purchase
consideration and the fair value of the acquired assets and
liabilities may be adjusted within 12 months from the acquisition
date. At the date of final measurement, adjustments are generally
made by restating comparative information previously determined
provisionally.
IM
Cannabis Corp.
Management’s Discussion and Analysis (Canadian dollars, in
thousands)
3.
On July 9, 2021,
the Company acquired all the issued and outstanding shares of MYM.
As a result, the company recognized goodwill of approximately
$41,192 and intangible assets, primarily brand name and customer
relationships, worth approximately $17,200 (based on a preliminary
purchase price allocation study). The goodwill arising on
acquisition is attributed to the expected benefits from the
synergies of the combination of the activities of the Company and
MYM, as well as value attributed to the assembled workforce, which
is included in goodwill. The goodwill recognized is not expected to
be deductible for income tax purposes.
The
Company recognized the fair value of the assets acquired and
liabilities assumed in the business combination according to a
provisional measurement. As of the date of the approval of the
Interim Financial Statements, a final valuation for the fair value
of the identifiable assets acquired and liabilities assumed by an
external valuation specialist has not been obtained. The purchase
consideration and the fair value of the acquired assets and
liabilities may be adjusted within 12 months from the acquisition
date. At the date of final measurement, adjustments are generally
made by restating comparative information previously determined
provisionally.
Liquidity and Capital Resources
For the
nine months ended September 30, 2021, the Company generated
revenues of $34,272, received $39,622 in net proceeds from issuance
of share capital at the Company’s financing round in May
2021, and $3,672 in proceeds from the exercises of Warrants, 2019
Compensation Options and Options.
In the
plan for use of available funds mentioned in the Company’s
prospectus, the Company have provided the following
information:
|
Uses of
Available Funds (Net)
|
|
|
CAPEX
Activities
|
4,340
|
|
M&A and
investments
|
14,880
|
|
Working
capital
|
11,750
|
|
General corporate
activities
|
8,652
|
|
TOTAL
|
39,622
|
The
Company believes that the generated cash flow form working capital
in the different jurisdictions on which it operates, as well as the
additional expected exercises of Unlisted Warrants and future
financing rounds will meet all its future requirements. In
evaluating its capital requirements, including the impact, if any,
on the Company from the COVID-19 pandemic, and the ability to fund
the execution of its strategy, the Company believes it has adequate
availability to meet its working capital and other operating
requirements, fund growth initiatives and capital expenditures,
settle its liabilities, and repay scheduled principal and interest
payments on debt for at least the next twelve months.
IM
Cannabis Corp.
Management’s Discussion and Analysis (Canadian dollars, in
thousands)
The
Company has ensured that it has access to public capital markets
through its CSE and NASDAQ listings and continues to review and
pursue selected external financing sources to ensure adequate
financial resources. These potential sources include, but are not
limited to, (i) obtaining financing from traditional or
non-traditional investment capital organizations and (ii) obtaining
funding from the sale of the Company’s securities. There can
be no assurance that we will gain adequate market acceptance for
our products or be able to generate sufficient positive cash flow
to achieve our business plans. We expect to continue funding these
purchases with our available cash, cash equivalents and short-term
investments. Therefore, we are subject to risks including, but not
limited to, our inability to raise additional funds through
financings to support our continued development, including capital
expenditure requirements, operating requirements and to meet our
liabilities and commitments as they come due. As at September 30,
2021, the Company had a working capital surplus of $43,568,
compared to working capital of $20,874 as at December 31, 2020. The
increase in working capital of $22,694 was primarily due to
increase in inventory, trade and other receivables, offset by trade
and other payables including purchase consideration payable. As of
September 30, 2021, the Company had a cash balance of
$17,116.
As at
September 30, 2021, the Group’s financial liabilities
consisted of accounts payable and other accounts payable which have
contractual maturity dates within one year. The Group manages its
liquidity risk by reviewing its capital requirements on an ongoing
basis. Based on the Group’s working capital position at
September 30, 2021, management considers liquidity risk to be
low.
As at
September 30, 2021, the Group has identified the following
liquidity risks related to financial liabilities
(undiscounted):
|
|
|
|
|
|
|
Lease
liabilities
|
$2,791
|
$11,023
|
$12,202
|
$3,224
|
The
maturity profile of the Company’s other financial liabilities
(trade payables, other account payable and accrued expenses, and
warrants) as of September 30, 2021 are less than one
year.
|
|
|
|
|
|
|
|
|
|
|
Debt
|
$5,863
|
$5,863
|
$-
|
$-
|
$-
|
|
Finance Lease Obligations
|
$29,495
|
$2,916
|
$5,863
|
$5,281
|
$15,435
|
|
Operating Leases
|
$122
|
$112
|
$10
|
$-
|
$-
|
|
Purchase Obligations
|
$9,959
|
$9,959
|
$-
|
$-
|
$-
|
|
Other Obligations
|
$-
|
$-
|
$-
|
$-
|
$-
|
|
Total Contractual Obligations
|
$45,439
|
$18,850
|
$5,873
|
$5,281
|
$15,435
|
IM
Cannabis Corp.
Management’s Discussion and Analysis (Canadian dollars, in
thousands)
The
Interim Financial Statements have been prepared on the basis of
accounting principles
applicable to a going concern, which assumes that the Company will
continue in operation for the foreseeable future and will be able
to realize its assets and discharge its liabilities in the normal
course of operations. The Interim Financial Statements do not
include any adjustments to the amounts and classification of assets
and liabilities that would be necessary should the Company be
unable to continue as a going concern. Such adjustments could be
material.
Share Capital
The
Company’s authorized share capital consists of an unlimited
number of Common Shares without par value, 67,838,041 of which were
issued and outstanding as at the date hereof.
The
Common Shares confer upon their holders the right to participate in
the general meeting with each Common Share having one voting right
on all matters. The Common Shares also allow holders to receive
dividends if and when declared and to participate in the
distribution of surplus assets in the case of liquidation of the
Company.
As of
September 30, 2021, the Company also has the following outstanding
securities which are convertible into, or exercisable or
exchangeable for, voting or equity securities of the Company:
5,555,745 Options, 1,840,690 Listed Warrants, 3,060,344 Unlisted
Warrants and 252,905 2019 Compensation Options.
Operating, Financing and Investing Activities
The
following table highlights the Company’s cash flow activities
for the nine and three months ended September 30, 2021 and 2020 and
year ended December 31, 2020:
|
|
For the Nine
Months Ended
September
30,
|
For the three months ended
September 30,
|
For the Year ended December 31,
|
|
Net cash provided by (used
in):
|
|
|
|
|
|
|
Operating
activities
|
$(39,134)
|
$(7,384)
|
$(16,123)
|
$(2,077)
|
$(7,919)
|
|
Investing
activities
|
$(1,930)
|
$(3,237)
|
$(6,001)
|
$(1,642)
|
$(4,075)
|
|
Financing
activities
|
$45,938
|
$6,238
|
$2,417
|
$251
|
$6,740
|
|
Effect
of foreign exchange
|
$3,357
|
$194
|
$2,773
|
$(16)
|
$213
|
|
Increase (Decrease)
in cash
|
$8,231
|
$(4,189)
|
$(16,934)
|
$3,484
|
$(5,041)
|
Operating
activities used cash of $39,134 and $7,384 for the nine months
ended September 30, 2021 and 2020, respectively. For the three
months ended September 30, 2021 and 2020, operating activities used
cash of $16,123 and $2,077, respectively. This variance is
primarily due to increase in the business activities of the Company
including corporate expenses for salaries, professional fees and
marketing expenses in Israel, Germany and Canada as well as costs
related to the NASDAQ listing and M&A processes. In the nine
months ended September 30, 2021, cash was primarily used to
increase operating activities in connection with the
Company’s operations in Canada and the NASDAQ listing and
M&A processes.
IM
Cannabis Corp.
Management’s Discussion and Analysis (Canadian dollars, in
thousands)
Investing
activities used cash of $1,930 and $3,237 for the nine months ended
September 30, 2021 and 2020, respectively. For the three months
ended September 30, 2021 and 2020, investing activities used cash
of $6,001 and $1,642, respectively. Cash was used primarily as
consideration for the M&A transaction costs and to enhance
production and purchase equipment for Focus, Adjupharm, Highland
and TJAC.
Financing
activities used cash of $45,938 and $6,238 for the nine months
ended September 30, 2021 and 2020, respectively. For the three
months ended September 30, 2021 and 2020, financing activities used
cash of $2,417 and $251, respectively. Most of the cash was derived
from the Company’s financing round in May 2021 in the amount
of $39,622.
Selected quarterly financial information
|
For the three
months ended
|
|
|
|
|
|
Net
Revenues
|
$14,393
|
$11,112
|
$8,767
|
$4,900
|
|
Net income
(Loss)
|
$(5,656)
|
$(5,089)
|
$4,715
|
$(19,976)
|
|
Basic net income
(Loss) per share (in CAD):
|
$(0.06)
|
$(0.10)
|
$0.11
|
$(0.50)
|
|
Diluted net loss
per share (in CAD):
|
$(0.18)
|
$(0.23)
|
$(0.06)
|
$(0.50)
|
|
For the three
months ended
|
|
|
|
|
|
Net
Revenues
|
$5,893
|
$3,757
|
$1,340
|
$2,479
|
|
Net income
(Loss)
|
$738
|
$(9,696)
|
$200
|
$1,693
|
|
Basic net income
(Loss) per share (in CAD):
|
$0.004
|
$(0.52)
|
$(0.003)
|
$0.015
|
|
Diluted net income
(Loss) per share (in CAD):
|
$0.004
|
$(0.52)
|
$(0.003)
|
$0.015
|
On a
quarterly basis, apart from the results of the first quarter of
2020 which were considered by the Company as preparation period for
successful delivery of medical cannabis products under the
Focus’ sales agreement to pharmacies, and the results of the
fourth quarter of 2020 which were affected by the COVID-19 outcomes
on the German market, the Company has consistently increased
revenues, which reflects the Company’s expansion
strategy.
Metrics and Non-IFRS Financial Measures
This
MD&A makes reference to certain non-IFRS financial measures
including “Gross Margin”, “EBITDA”, and
“Adjusted EBITDA”. These measures are not recognized
measures under IFRS and do not have a standardized meaning
prescribed by IFRS and are therefore unlikely to be comparable to
similar measures presented by other companies. Rather, these
measures are provided as additional information to complement those
IFRS measures by providing further understanding of our results of
operations from management’s perspective. Accordingly, these
measures should neither be considered in isolation nor as a
substitute for analysis of our financial information reported under
IFRS.
IM
Cannabis Corp.
Management’s Discussion and Analysis (Canadian dollars, in
thousands)
Management
defines EBITDA as income earned or lost from operations, as
reported, before interest, tax, depreciation and amortization.
Adjusted EBITDA is defined as EBITDA, adjusted by removing other
non-recurring or non-cash items, including the unrealized change in
fair value of biological assets, realized fair value adjustments on
inventory sold in the period, share-based compensation expenses,
and revaluation adjustments of financial assets and liabilities
measured on a fair value basis. Management believes that Adjusted
EBITDA is a useful financial metric to assess its operating
performance on a cash adjusted basis before the impact of
non-recurring or non-cash items. The Company defines gross margin
as the difference between revenue and cost of goods sold divided by
revenue (expressed as a percentage), prior to the effect of a fair
value adjustment for inventory and biological assets.
These
non-IFRS financial measures can provide investors with supplemental
measures of our operating performance and thus highlight trends in
our core business that may not otherwise be apparent when relying
solely on IFRS measures. We also believe that securities analysts,
investors and other interested parties frequently use non-IFRS
financial measures in the evaluation of issuers. Our management
also uses these non-IFRS financial measures in order to facilitate
operating performance comparisons from period to period, to prepare
annual operating budgets and forecasts and to determine components
of management compensation. As required by Canadian securities
laws, we reconcile these non-IFRS financial measures to the most
comparable IFRS measures.
|
|
For the Nine
Months Ended
September
30,
|
For the three months ended
September 30,
|
For the Year ended
December 31,
|
|
|
|
|
|
|
|
|
|
Operating
Loss
|
$(26,667)
|
$(1,862)
|
$(14,245)
|
$(671)
|
$(8,245)
|
$(10,275)
|
|
Depreciation &
Amortization
|
$3,604
|
$672
|
$1,961
|
$244
|
$930
|
$601
|
|
EBITDA
|
$(23,063)
|
$(1,190)
|
$(12,284)
|
$(427)
|
$(7,315)
|
$(9,674)
|
|
IFRS Biological
assets fair value adjustments, net
|
$2,124
|
$(3,943)
|
$1,355
|
$(19)
|
$(1,659)
|
$384
|
|
Share-based
payments
|
$5,354
|
$2,131
|
$3,351
|
$704
|
$3,382
|
$2,677
|
|
Non-recurring costs
related to the RTO
|
-
|
-
|
-
|
-
|
-
|
$3,632
|
|
Costs related to
the NASDAQ listing
|
$1,261
|
-
|
-
|
-
|
$175
|
-
|
|
Other Non-recurring
costs
|
$570
|
$525
|
$570
|
-
|
$520
|
$1,167
|
|
Adjusted
EBITDA (Non-IFRS)1
|
$(13,754)
|
$(2,477)
|
$(7,008)
|
$258
|
$(4,897)
|
$(1,814)
|
Notes:
(1)
Acquisition costs,
in the amount of $4,327 and $1,620 for the nine and three months
ended September 30, 2021, respectively, have not been adjusted in
the above-mentioned table. Had these non-operational acquisition
costs been adjusted, the Company’s Adjusted EBITDA for the
nine and three months ended September 30, 2021 would have been
$(9,427) and $(5,388), respectively.
IM
Cannabis Corp.
Management’s Discussion and Analysis (Canadian dollars, in
thousands)
Adjusted
EBITDA for the nine months ended September 30, 2021 and 2020 was
$(13,754) and $(2,477), respectively, representing a decrease of
$11,277. Adjusted EBITDA for the three months ended September 30,
2021, and 2020 was $(7,008) and $258, respectively, representing a
decrease of $7,266. The Company’s Adjusted EBITDA for the
nine months ended September 30, 2021 decreased primarily due to the
previously disclosed delays in contracted shipments to Germany from
its primary supply partner as well as temporary production
constraints in Canada during the second quarter of 2021 and
integration costs for acquisitions in Canada and Israel. Additional
impact on the adjusted EBITDA derived from general and
administrative costs mainly attributable to the growing corporate
activities in Israel, Germany, and Canada, professional services
derived from legal fees and other consulting services, M&A
transaction costs, salaries to employees and increased insurance
costs upon listing on NASDAQ. Adjusted EBITDA is expected to climb
with the full integration of Trichome and MYM as well as the
consolidation of the newly acquired retail activities in
Israel.
Contingent Liabilities and Commitments
(i) Rental
Liabilities
In
August 2010, Focus signed an agreement with a farmer, located in
the south of Israel (the “Farmer”), according to which,
Focus and the Farmer agreed to jointly operate an area of 7,000
square meters (the “Focus Facility”) for the
cultivation and processing of medical cannabis (the
“Venture”). For the purpose of this Venture, the
parties agreed to operate under the operation of Focus. As part of
the agreement, 26% of the share capital of Focus was allocated to
the Farmer.
On
December 1, 2016, Focus signed an additional agreement with the
Farmer, extending the Focus Facility by an additional area of 6,000
square meters for the cultivation and processing of medical
cannabis.
On
October 29, 2019, Focus signed with the Farmer an additional
agreement for the extension of the Focus Facility by an additional
area of 7,500 square meters for the cultivation and processing of
medical cannabis.
In
April 2021, IMC entered into a lease agreement with Kibutz Glil Yam
for the Company’s head office.
In July
2021, IMC entered into a sublease agreement for facility space to
perform certain activities acquired under the Panaxia
Transaction.
Through
the Trichome Transaction, the Company acquired two cultivation and
processing facilities in Ontario, Canada, the leases of which
expire in 2026 and 2033.
IM
Cannabis Corp.
Management’s Discussion and Analysis (Canadian dollars, in
thousands)
(ii) Class
Action T.Z. 35676-08-19
On
August 19, 2019, a motion was filed for approval of a class action
(the “Motion”) against 17 companies (the
“Companies”) operating in the field of medical cannabis
in Israel, including Focus. The applicant’s argument is that
the Companies did not accurately mark the concentration of active
ingredients in their products. The personal suit sum for every
class member stands at NIS 15,585 ($5,900) and the total
amount of the class action suit is estimated at NIS 686,000
($259,000). On June 2, 2020, the Companies submitted their response
to the Motion. The Companies argue in their response that the
threshold conditions for approval of a class action were not met,
since there is no reasonable possibility that the causes of action
in the Motion will be decided in favor of the class group. On July
3, 2020, the applicant submitted his response to the
Companies’ response. On July 5, 2020 the applicant was absent
from the hearing. As a result, on July 23, 2020, the Companies
filed an application for a ruling of expenses, which received a
response from the applicant on August 12, 2020, asking to decline
this request. On September 21, 2020, the court ruled that the
applicant would pay the Companies’ expenses amount of NIS
750. On July 14, 2021 a hearing was held. The court recommended the
parties to negotiate independently to avoid litigation, and if
negotiations fail, then to begin mediation proceedings. The parties
agreed to follow the court’s recommendations, though the
negotiations between the parties have not yet begun.
At this
preliminary stage, based on the opinion of its legal counsel,
Focus’ management cannot assess the chances of approval of
the Motion nor the chances of the claims under the Motion being
accepted if the Motion is approved. Therefore, no provision has
been recorded in respect thereof.
(iii) Supreme
Court of Justice 2335/19
On
October 6, 2019, Focus received a decision regarding a petition
that was filed against the MOH, concerning the new regulatory
framework of the cannabis market and demanding that the court
resolve as follows:
●
that the MOH
immediately suspend the implementation of the new regulation that
harms, disproportionally, the medical cannabis
patients;
●
that the
implementation of the new regulation, as is, would cause violation
of constitutional rights of the medical cannabis patients;
and
●
that the MOH amend
the flaws of the new regulation, prior to becoming effective, and
to establish new regulations regarding labeling and use of
pesticides.
According
to the decision, Focus was attached to the proceedings and filed
its response on November 12, 2019.
On
March 8, 2020, the court decided to extend the validity of the
interim injunction, so that the medical cannabis use licenses,
which were extended under the decision, would continue to be valid
until May 15, 2020, or 10 days after the date the MOH comes to a
conclusion regarding the price control of medical cannabis
products, whichever comes first, subject to another court
decision.
IM
Cannabis Corp.
Management’s Discussion and Analysis (Canadian dollars, in
thousands)
The
court also decided that if a further extension of the period of the
interim injunction is granted beyond May 15, 2020, to the extent
required, it would be subject to medical surveillance by the
attending physician, that his details of which were included in the
patient’s existing use license.
On
October 29, 2020, the respondents represented by the State
Attorney’s Office filed an update notice stating that the
Appeals Committee unanimously decided against imposing price
controls on medical cannabis products and that the Prices Committee
would hold a follow-up hearing in four months. The respondents also
requested to update the Court again in two months.
On
November 25, 2020, the petitioner submitted their response to the
respondents’ update notice.
On
March 25, 2021, the respondents represented by the State
Attorney’s Office filed an updating notice stating that the
Prices Committee had come to a decision against imposing price
controls on medical cannabis products. However, the Prices
Committee announced that it will issue a request for information to
the corporations engaged in the medical cannabis market and assess
the market every six months. Following the aforementioned, the
respondents represented by the State Attorney’s Office
believe that the appeal should be rejected and the interim
injunction should be canceled. On April 13, 2021, three of the
respondents filed a response to the court, requesting to reject the
appeal and to cancel the interim injunction.
On
April 25, 2021, the petitioner filed a response to the update
notice to the court, objecting to the position of the respondents
represented by the State Attorney’s Office, requesting the
court to resolve as requested in the petition and grant the
requested remedies to the petitioner. On July 6, 2021, the
petitioner filed an urgent request to the court, to issue orders to
the respondents represented by the State Attorney’s Office,
to request information from corporations engaged in the medical
cannabis market in order to continue the examination of the market,
according to the Prices Committee’s announcement mentioned
above, and requested the court to reschedule the hearing set to
occur on September 19, 2021, to an earlier date. The
petitioner’s request was rejected by the court on July 7,
2021, and on September 19, 2021, a hearing was held. Currently, the
court has not yet issued any ruling.
Based
on the opinion of its legal counsel, Focus’ management
estimates that the chances of the petition to be accepted by the
court are 50% and at this stage no adverse outcome is expected;
therefore, no provision has been recorded in respect
thereof.
(iv) Planning
and Construction 66813-06-21
On July
11, 2021, the Company was informed that a claim (the
“Construction Proceedings”) was filed by the municipal
committee presiding over planning and construction in southern
Israel (the “Construction Committee”) against Focus,
Focus’ directors and officers, and certain landowners,
including Oren Shuster and Rafael Gabay, claiming for inadequate
permitting for construction relating to the Focus Facility
(“Construction Allegations”). A hearing was set to
December 1, 2021.
At this
preliminary stage, based on the opinion of its legal counsel,
Focus’ management cannot assess the chances of the claim
advancing or the potential outcome of the Construction
Proceedings.
IM
Cannabis Corp.
Management’s Discussion and Analysis (Canadian dollars, in
thousands)
Off-Balance Sheet Arrangements
IMCC
had no off-balance sheet arrangements as at September 30,
2021.
Transactions with Related Parties
Trichome,
through a management service agreement, provided investment
management services to the Fund during the quarter ended September
30, 2021. Under IFRS 10, the Fund is an equity accounted investment
and therefore is not consolidated with the results of the
Company.
Other
than the investment management activities noted above, the Company
had no other transactions with related parties outside of the group
except those pertaining to transactions with key management
personnel and shareholders in the ordinary course of their
employment or directorship. Transactions with related parties for
the sale of Focus due to the restructuring process were adjusted in
the Company’s consolidated financial statements following the
application of IFRS 10.
Proposed Transactions
There
are no proposed transactions as at the date of this MD&A that
have not been disclosed.
Critical Accounting Estimates
In the
process of applying the significant accounting policies, the Group
has made the following judgments which have the most significant
effect on the amounts recognized in the financial
statements:
Determining the fair value of share-based payment
transactions
The
fair value of share-based payment transactions is determined upon
initial recognition by an acceptable option pricing model. The
inputs to the model include share price, exercise price and
assumptions regarding expected volatility, expected life of the
share options and expected dividend yield.
Discount rate for a lease liability
When
the Company is unable to readily determine the discount rate
implicit in a lease in order to measure the lease liability, the
Company uses an incremental borrowing rate. That rate represents
the rate of interest that the Company would have to pay to borrow
over a similar term and with similar security, the funds necessary
to obtain an asset of similar value to the right-of-use asset in a
similar economic environment. When there are no financing
transactions that can serve as a basis, the Company determines the
incremental borrowing rate based on its credit risk, the lease term
and other economic variables deriving from the lease
contract’s conditions and restrictions. The rates at which
the Company can borrow will also vary based on the jurisdiction of
the leased property, whether it be Israel, Germany, or Canada. In
certain situations, the Company is assisted by an external
valuation expert in determining the incremental borrowing
rate.
IM
Cannabis Corp.
Management’s Discussion and Analysis (Canadian dollars, in
thousands)
b.
Estimates and assumptions
The
preparation of the financial statements requires management to make
estimates and assumptions that have an effect on the application of
the accounting policies and on the reported amounts of assets,
liabilities, revenues and expenses. Changes in accounting estimates
are reported in the period of the change in estimate.
The key
assumptions made in the financial statements concerning
uncertainties at the reporting date and the critical estimates
computed by the Group that may result in a material adjustment to
the carrying amounts of assets and liabilities within the next
financial year are discussed below.
Assessment of going concern
The use
of the going concern basis of preparation of the financial
statements. At each reporting period, management assesses the basis
of preparation of the financial statements. These financial
statements have been prepared on a going concern basis in
accordance with IFRS. The going concern basis of presentation
assumes that the Company will continue its operations for the
foreseeable future and be able to realize its assets and discharge
its liabilities and commitments in the normal course of
business.
In
arriving at this determination, the Company has undertaken a
thorough review of the Group’s cash flow forecast and
potential liquidity risks. Cash flow projections have been prepared
which show that the Group’s operations will be cash
generative during the period of at least 12 months from the date of
approval of the consolidated financial statements.
Biological assets and inventory
In
calculating the value of the biological assets and inventory,
management is required to make several estimates, including
estimating the stage of growth of the cannabis up to the point of
harvest, harvesting costs, selling costs, average or expected
selling prices and list prices, expected yields for the cannabis
plants, and oil conversion factors. The valuation of
work-in-process and finished goods also requires the estimate of
conversion costs incurred, which become part of the carrying amount
for the inventory. The Company must also determine if the cost of
any inventory exceeds its net realizable value, such as cases where
prices have decreased, or inventory has spoiled or has otherwise
been damaged. See Note 4 of the Interim Financial Statements for
further information.
Business combinations
In
determining the fair value of all identifiable assets acquired and
liabilities assumed, the most significant estimates generally
relate to contingent consideration and intangible assets.
Management exercises judgment in estimating the probability and
timing of when earn-outs are expected to be achieved, which is used
as the basis for estimating fair value. Identified intangible
assets are fair valued using appropriate valuation techniques which
are generally based on a forecast of the total expected future net
cash flows of the acquiree. Valuations are highly dependent on the
inputs used and assumptions made by management regarding the future
performance of these assets and any changes in the discount rate
applied.
IM
Cannabis Corp.
Management’s Discussion and Analysis (Canadian dollars, in
thousands)
Impairment of property, plant and equipment and finite life
intangible assets
The
Company assesses impairment of property, plant and equipment and
finite life intangible assets when an impairment indicator arises
(e.g., change in use or discontinued use, obsolescence or physical
damage). When the asset does not generate cash inflows that are
largely independent of those from other assets or group of assets,
the asset is tested at the cash generating unit (“CGU”)
level. In assessing impairment, the Company compares the carrying
amount of the asset or CGU to the recoverable amount, which is
determined as the higher of the asset or CGU’s fair value
less costs of disposal and its value-in-use. Value-in-use is
assessed based on the estimated future cash flows, discounted to
their present value using a pre-tax discount rate that reflects
applicable market and economic conditions, the time value of money
and the risks specific to the asset. An impairment loss is
recognized whenever the carrying amount of the asset or CGU exceeds
its recoverable amount and is recorded in the consolidated
statements of comprehensive loss.
Impairment of intangible assets with indefinite life and
goodwill
Goodwill
and intangible assets with an indefinite life or not yet available
for use are tested for impairment annually, and whenever events or
circumstances that make it more likely than not that an impairment
may have occurred, such as a significant adverse change in the
business climate or a decision to sell or dispose all or a portion
of a reporting unit. Finite life intangible assets are tested
whenever there is an indication of impairment. Goodwill and
indefinite life intangible assets are tested for impairment by
comparing the carrying value of each CGU containing the assets to
its recoverable amount. Goodwill is allocated to CGUs or groups of
CGU’s for impairment testing based on the level at which it
is monitored by management, and not at a level higher than an
operating segment. Goodwill is allocated to those CGUs or groups of
CGUs expected to benefit from the business combination from which
the goodwill arose, which requires the use of judgment. An
impairment loss is recognized for the amount by which the
CGU’s carrying amount exceeds it recoverable amount. The
recoverable amounts of the CGUs’ assets have been determined
based on either fair value less costs of disposal or value-in-use
method. There is a material degree of uncertainty with respect to
the estimates of the recoverable amounts of the CGU, given the
necessity of making key economic assumptions about the future.
Impairment losses recognized in respect of a CGU are first
allocated to the carrying value of goodwill and any excess is
allocated to the carrying value of assets in the CGU. Any
impairment is recorded in profit and loss in the period in which
the impairment is identified. A reversal of an asset impairment
loss is allocated to the assets of the CGU on a pro rata basis. In
allocating a reversal of an impairment loss, the carrying amount of
an asset shall not be increased above the lower of its recoverable
amount and the carrying amount that would have been determined had
no impairment loss been recognized for the asset in prior period.
Impairment losses on goodwill are not subsequently
reversed.
Legal claims
In
estimating the likelihood of legal claims filed against certain
entities of the Group, the Company’s management rely on the
opinions of the respective legal counsel of each relevant entity of
the Group. These estimations are based on each legal
counsel’s best professional judgment, taking into account the
stage of proceedings and legal precedents in respect of the
different issues. Since the outcome of the claims may be determined
in courts, the results could differ from these
estimations.
IM
Cannabis Corp.
Management’s Discussion and Analysis (Canadian dollars, in
thousands)
Deferred tax assets
Deferred
tax assets are recognized for unused carryforward tax losses and
deductible temporary differences to the extent that it is probable
that taxable profit will be available against which the losses can
be utilized. Significant management judgment is required to
determine the amount of deferred tax assets that can be recognized,
based upon the timing and level of future taxable profits, its
source and the tax planning strategy.
Valuation of loans receivable
For
loans measured at amortized cost or at Fair Value Through Profit or
Loss (“FVTPL”) under IFRS 9 Financial Instruments (“IFRS
9”), judgment is used by the Company in determining the fair
value of the loan at inception of the lending arrangement and at
each reporting period. The fair value of the loan at any given
point in time is calculated based on the present value of estimated
future loan payments, discounted using an interest rate that would
be charged by another market participant for a financing
arrangement with similar characteristics. Judgment is used by the
Company in determining what the interest rate would be for sourcing
a similar financing arrangement in the market. This can lead to
material fair value gains or losses on loans held at
FVTPL.
Derecognition and modification of loans receivable
The
Company uses judgment in determining whether the change in the
terms of the lending arrangement qualifies as a derecognition of
the loan or a modification of the loan under IFRS 9. Depending on
the Company’s judgment, the manner in which the loan is
treated, be it a modification or a settlement, can result in
materially different results in interest revenue or other income.
If there is a modification in a lending arrangement subsequent to
initial recognition, the Company also reassesses the need to modify
the expected credit loss associated with the loan.
Share-based payments
The
Company uses the Black-Scholes option pricing model in determining
the fair value of Options issued to employees. In estimating fair
value, the Company is required to make certain assumptions and
estimates such as the expected life of the options, volatility of
the Company’s future share price, the risk-free rate, future
dividend yields and estimated forfeiture rates at the initial grant
date.
Equity accounted investees
Significant
judgment is used by the Company in assessing control of the
Company’s investment in its equity accounted investee –
the Fund. Although not holding more than a 20% stake in the Fund,
the Company concluded that significant influence exists under IFRS
10 based on the Company’s management of day-to-day operations
of the business and overall investment management.
Estimated useful lives and depreciation/amortization of property
and equipment, as well as intangible assets
IM
Cannabis Corp.
Management’s Discussion and Analysis (Canadian dollars, in
thousands)
Depreciation
and amortization of property and equipment, as well as intangible
assets, are dependent upon estimated useful lives which are
determined through the exercise of judgment. Estimated useful lives
are assessed at the end of each reporting period for any changes in
the expected life of the asset and consumption of economic benefits
from the use of the asset. Amortization as well as depreciation
commences when the asset is first put into use. The expected life
of any intangible assets with a finite life are assessed at the end
of each reporting period.
Leases
Judgment
is used in determining the value of the Company’s
right-of-use assets and lease liabilities. The value determined for
the Company’s right-of-use assets and lease liabilities can
be materially different based on the discount rate selected to
present value the future lease payments as well as the likelihood
of the Company exercising extensions, termination, and/or purchase
options. The discount rate used to present value the future lease
payments over the life of the lease is based on the Company’s
incremental borrowing rate at inception of the lease. This rate is
determined by the Company using judgment.
In
determining the value of the Company’s right-of-use assets
and lease liabilities, the Company assesses future business plans
to determine whether to include certain extension options noted in
the lease agreement.
If
there is no interest rate implicit in the lease agreement, the
Company uses a discount rate that would be charged to a similar
borrower, with similar risk characteristics, in a mortgage loan to
purchase the leased facility. This discount rate is used to present
value the future lease payments in determining the right-of-use
asset and lease liability values at inception of the
leases.
Revenue recognition
Under
IFRS 15 Revenue from Contracts with Customers, judgment is required
in recognizing revenue when variable consideration is present in a
contract. In certain supply agreements, the Company stands ready to
accept returns on cannabis sales, indicating the possibility of
variable consideration.
Judgment
is used by the Company in determining which of the above two
methods of revenue recognition should be used when recognizing
revenue from cannabis sales. Moreover, estimates are used by the
Company in determining the amount of revenue to recognize upon
delivery and acceptance of cannabis inventory to a
customer.
Risk Factors
The
Company has implemented risk management governance processes that
are led by the board of directors, with the active participation of
management, and updates its assessment of its business risks on an
annual basis. Notwithstanding, it is possible that the Company may
not be able to foresee all the risks that it may have to face. The
market in which IMCC currently competes is complex, competitive and
changing rapidly. Sometimes new risks emerge, and management may
not be able to predict all of them or be able to predict how they
may cause actual results to be different from those contained in
forward looking statements. Readers of this MD&A should not
rely upon forward looking statements as a prediction of future
results.
IM
Cannabis Corp.
Management’s Discussion and Analysis (Canadian dollars, in
thousands)
The
following risk factors have been identified by
management:
(i) General
Business Risk and Liability
Given
the nature of the Group’s business, it may from time to time
be subject to claims or complaints from investors or others in the
normal course of business. The legal risks facing the Group, its
directors, officers, employees or agents in this respect include
potential liability for violations of securities laws, breach of
fiduciary duty or misuse of investors’ funds. Some violations
of securities laws and breach of fiduciary duty could result in
civil liability, fines, sanctions, or the suspension or revocation
of the Group’s right to carry on its existing business. The
Group may incur significant costs in connection with such potential
liabilities.
(ii) Consolidation
of Focus Financial Results under IFRS 10 and Maintenance of Common
Control
The
Company complies with IFRS 10, which applies a single consolidation
model using a definition of “control” that requires an
investor (as defined in IFRS 10) to consolidate an investee (as
defined in IFRS 10) where: (i) the investor has power over the
investee; (ii) the investor has exposure or rights to variable
returns from involvement with the investee; and (iii) the investor
can use its power over the investee to affect the amount of the
investor’s returns.
Subsequent
to the IMC Restructuring, the Company analyzed the terms of the
contractual agreements with Focus (including the Commercial
Agreements and the Focus Agreement) in accordance with IFRS 10 to
conclude whether it should continue to consolidate the accounts of
Focus in its financial statements.
Under
IFRS 10, consolidation occurs when an investor can exercise control
over an investee. Control is achieved through voting rights or
other evidence of power. Where there are no direct holdings, under
IFRS 10, an investor (as defined in IFRS 10) should consider other
evidence of power and ability to unilaterally direct an
investee’s (as defined in IFRS 10) relevant activities. In
view of the contractual agreements and the guidance in IFRS 10,
notwithstanding that the Company has no direct or indirect
ownership of Focus, it has sufficient rights to unilaterally direct
the relevant activities (a concept known as “de facto
control”), mainly due to the following:
(a)
the Company
receives economic benefits from Focus (and the terms of the
Commercial Agreements cannot be changed without the approval of the
Company);
(b)
the Company having
the option to purchase the divested 74% interest in Focus held by
Oren Shuster, the Chief Executive Officer, director and a promoter
of the Company, and Rafael Gabay, a consultant promoter and former
director of the Company;
(c)
Messrs. Shuster and
Gabay each being a director of Focus (while Mr. Shuster
concurrently being a director, officer and substantial shareholder
of the Company and Mr. Gabay concurrently being a substantial
shareholder of the Company); and
(d)
the Company
provides management and support activities to Focus through the
Services Agreement.
IM
Cannabis Corp.
Management’s Discussion and Analysis (Canadian dollars, in
thousands)
Accordingly,
under IFRS 10, the Company has “de facto control” over
Focus, and therefore consolidates the financial results of Focus in
the Company’s financial statements.
Any
failure of the Company or Messrs. Oren Shuster and Rafael Gabay to
maintain “de facto control” over Focus as defined under
IFRS 10 could alter the Company’s consolidation model,
potentially resulting in a material adverse effect on the business,
results of operations and financial condition of the
Company.
(i) Ownership
of Focus
There
is a risk that regulatory authorities in Israel may view the
Company as the deemed owner of more than 5% of Focus and/or
determine that the Company is in contravention of Israeli cannabis
regulations. Namely, prior approval of the IMCA is required for any
shareholder owning 5% or more of an Israeli company licensed to
engage in cannabis-related activities in Israel. Any contravention
of Israeli cannabis regulations could jeopardize the good standing
of the Focus License. Such a determination may adversely affect the
Group’s ability to conduct sales and marketing activities and
could have a material adverse effect on the Group’s business,
operating results or financial condition.
(ii) Possible
Direct Involvement in the Israeli Cannabis Industry
Neither
the Company nor any of its subsidiaries currently hold, directly or
indirectly, any licenses to engage in the propagation, cultivation,
production, processing, distribution or sale of medical cannabis
products in Israel (the “Cannabis Activities”).
According to current Israeli regulatory medical cannabis framework,
any engagement in Cannabis Activities requires receiving the
applicable license from the IMCA, an agency operated by the MOH,
which requires, among other things, pre-approvals by the IMCA (the
“IMCA Pre-Approval Requirement”) of the directors,
officers and shareholders holding 5% or more of the shares of the
license applicant (“Material Holders”), and of all
directors, officers and shareholders that become Material Holders
(“Future Material Holders”) following the grant of the
applicable license. Therefore, any direct engagement of the Company
in Cannabis Activities will require the aforementioned approvals by
the IMCA and will apply, upon such approval and granting of a
license, limitations on future security holdings, as described
above.
Furthermore,
due to the uncertainty related to the broad administrative
discretion over the activities in the medical cannabis industry in
Israel granted to the IMCA, the IMCA Pre-Approval Requirement and
the restrictions on security holdings discussed in this risk factor
may apply to the Company and its shareholders by virtue of a
subsidiary or investee engaging in Cannabis Activities. In such
cases, any failure of the Company or its shareholders to comply
with the IMCA Pre-Approval Requirement may impact the Group’s
ability to continue operating in compliance with any licenses to
engage in Cannabis Activities or to renew such licenses. Any
inability of the Group to maintain licenses for Cannabis Activities
in good standing may result in a material adverse effect on the
Group’s business, financial condition, results of operations
and prospects.
Following
the anticipated completion of the acquisition of the Panaxia
IMC-GDP License pursuant to the Panaxia Transaction, the Pharm
Yarok Transaction and the Vironna Transaction, the Company will be
considered to be engaging in Cannabis Activities under Israeli
cannabis regulations, which the Company expects will necessitate
compliance with the IMCA Pre-Approval Requirement. In addition, the
IMCA Pre-Approval Requirement may apply to the Company, as the sole
shareholder of IMC Holdings, and may require all of its directors,
officers, Material Holders, and Future Material Holders to comply
with the IMCA Pre-Approval Requirement following the
Company’s anticipated receipt of the Panaxia IMC-GDP License
and the holdings of Pharm Yarok Group and Vironna.
IM
Cannabis Corp.
Management’s Discussion and Analysis (Canadian dollars, in
thousands)
Although
the Company believes that it will meet the IMCA Pre-Approval
Requirement in connection with its future engagement in Cannabis
Activities, including the anticipation of receiving the Panaxia
IMC-GDP License, and the Panaxia Pharmacy Licenses if acquired upon
exercising the Panaxia Option, the completion of the Pharm Yarok
Transaction and the Vironna Transaction, there can be no guarantee
that such requirements will be met or that the Company will be able
to comply on an ongoing basis. If any of the directors, officers,
Material Holders or Future Material Holders of IMC Holdings or the
Company, as applicable, fail to comply with the IMCA Pre-Approval
Requirement while IMC Holdings or the Company, as applicable, are
engaging in Cannabis Activities, any licenses to engage in Cannabis
Activities then acquired may be revoked, suspended or otherwise
affected. Any change to the status of any license as a result of
failing to comply with the IMCA Pre-Approval Requirement, including
Future Material Holders failing to obtain sufficient approvals, may
result in a material adverse effect on the Company’s
business, financial condition, results of operations and
prospects.
(iii) Limited
Operating History
The
Company did not generate revenue from the sale of cannabis products
until late 2019. The Company is therefore subject to many of the
risks common to early-stage enterprises, including
under-capitalization, cash shortages, limitations with respect to
personnel, financial, and other resources and lack of revenues.
There is no assurance that the Company will be successful in
achieving a return on shareholders’ investment and the
likelihood of success must be considered in light of the early
stage of operations.
(iv) Negative
Cash Flows
During
the nine months Ended September 30, 2021, the Company had negative
cash flows from operating activities. Although the Company expects
to generate positive cash flows from its future operating
activities, there is no assurance that it will achieve this
objective. If operational cash flows continue to be negative, the
Company may be required to fund future operations with alternative
financing options such as offerings of securities. Continued
negative cash flow may restrict the Company’s ability to
pursue its business objectives.
(v) Additional
Financing
There
is no assurance that the Company will be able to secure the funds
necessary to implement its strategies. Additional debt incurred by
the Company from engagements such as major acquisitions may cause
the Company’s debt level to increase and result in
difficulties in completing or negotiating future debt financings.
Any triggering of credit defaults or failure to raise capital by
the Company may cause significant delays in carrying out business
objectives or result in a material adverse effect on the
Company’s business, financial condition, operational results
and prospects.
IM
Cannabis Corp.
Management’s Discussion and Analysis (Canadian dollars, in
thousands)
(vi) No
Control over Cannabis Operations of Investees
The
Company’s investees generally have the power to determine the
manner in which their respective businesses are developed, expanded
and operated. The interests of the Company and its investees may
not always be aligned. As a result, the cash flows of the Company
are dependent upon the activities of its investees, which creates
the risk that at any time those investees may: (i) have business
interests or targets that are inconsistent with those of the
Company; (ii) take action contrary to the Company’s policies
or objectives; (iii) be unable or unwilling to fulfill their
obligations under their agreements with the Company; or (iv)
experience financial, operational or other difficulties, including
insolvency, which could limit or suspend an investee’s
ability to perform its obligations under its agreements with the
Company. The Company must rely on the accuracy and timeliness of
the disclosure and information it receives from its investees. If
the information contains material inaccuracies or omissions, the
Company’s ability to accurately forecast or achieve its
stated objectives may be materially impaired. Failure to receive
Company’s entitlements pursuant to the agreements it has
entered into may have a material adverse effect on the
Company.
(vii) Compliance
with Laws
The
Group’s and its investees’ operations are subject to
various laws, regulations and guidelines. The Group endeavors to
and cause its investees to comply with all relevant laws,
regulations and guidelines. However, there is a risk that the
Group’s and its investees’ interpretation of laws,
regulations and guidelines, including, but not limited to the
Cannabis Act (Canada) (the
“Cannabis Act”), the regulations thereunder and
applicable stock exchange rules and regulations, may differ from
each other, and the Group’s and its investees’
operations may not be in compliance with such laws, regulations and
guidelines. In addition, achievement of the Group’s business
objectives is contingent, in part, upon compliance with regulatory
requirements enacted by governmental authorities and, where
necessary, obtaining regulatory approvals. The impact of regulatory
compliance regimes, any delays in obtaining, or failure to obtain
regulatory approvals required by the Group or its investees may
significantly delay or impact the development of the Group’s
business and operations and could have a material adverse effect on
the business, results of operations and financial condition of
Group. Any potential noncompliance could cause the business,
financial condition and results of operations of the Group to be
adversely affected. Further, any amendment to or replacement of the
Cannabis Act or other applicable rules and regulations governing
the activities of the Group, or its investees may cause adverse
effects to Group’s operations. The risks to the business of
Group and its investees associated with the decision to amend or
replace the Cannabis Act and subsequent regulatory changes, could
reduce the addressable market for the Group’s or the
investees’ products and could materially and adversely affect
the business, financial condition and results of operations of the
Group.
The
Group and its investees incur ongoing costs and obligations related
to regulatory compliance. Failure to comply with applicable laws
and regulations may result in enforcement actions thereunder,
including orders issued by regulatory or judicial authorities
causing operations to cease or be curtailed, and may include
corrective measures requiring capital expenditures or remedial
actions. The Group may be liable for civil or criminal fines or
penalties imposed for violations of applicable laws or regulations.
Amendments to current laws, regulations and permitting
requirements, or more stringent application of existing laws or
regulations, may have a material adverse impact on the Group and/or
its investees, resulting in increased capital expenditures or
production costs, reduced levels of cannabis production or
abandonment or delays in the development of facilities which could
have a material adverse effect on the business, results of
operations and financial condition of the Group.
IM
Cannabis Corp.
Management’s Discussion and Analysis (Canadian dollars, in
thousands)
The
introduction of new tax laws, regulations or rules, or changes to,
or differing interpretations of, or application of, existing tax
laws, regulations or rules in any of the countries in which the
Company invests could result in an increase in the Company’s
taxes, or other governmental charges, duties or impositions. No
assurance can be given that new tax laws, regulations or rules will
not be enacted or that existing tax laws, regulations or rules will
not be changed, interpreted or applied in a manner which could
result in the Company’s profits being subject to additional
taxation or which could otherwise have a material adverse effect on
the Company.
(viii) Regulation
of the Cannabis Industry
The
cannabis-related business and activities of the Group are heavily
regulated in all jurisdictions where it carries on business. The
Group’s operations are subject to various laws, regulations
and guidelines by governmental authorities, particularly the MOH
and The Federal Opium Agency of
Germany’s Federal Institute for Drugs and Medical Devices
(the “BfArM”), relating to the manufacturing,
marketing, management, transportation, distribution, storage, sale,
pricing and disposal of medical cannabis and cannabis oil, and also
including laws and regulations relating to health and safety,
insurance coverage, the conduct of operations and the protection of
the environment. In Canada, the regulation on access to cannabis
for medical purposes was established by Health Canada in July 2001.
The Cannabis Act, which
came into force on October 17, 2018, currently governs the
production, sale and distribution of medical and recreational
cannabis and related oil extracts in Canada and provides for the
regulation by the Canadian federal government of, among other
things, the commercial cultivation and processing of cannabis for
recreational purposes. The Cannabis Act also authorizes Canadian
provinces and territories to regulate other aspects of recreational
cannabis, such as distribution, sale, minimum age requirements,
places where cannabis can be consumed, and a range of other
matters. The governments of each Canadian province and territory
have implemented regulatory regimes for the distribution and sale
of cannabis for recreational purposes.
Laws
and regulations, applied generally, grant government agencies and
self-regulatory bodies broad administrative discretion over the
activities of the Group, including the power to limit or restrict
business activities as well as impose additional disclosure
requirements on the Group’s products and services.
Achievement of the Group’s business objectives are
contingent, in part, upon compliance with regulatory requirements
enacted by these governmental authorities and obtaining all
regulatory approvals, where necessary, for the production and sale
of its products.
The
Group cannot predict the time required to secure all appropriate
regulatory approvals for its products, or the extent of testing and
documentation that may be required by governmental authorities. Any
delays in obtaining, or failure to obtain regulatory approvals
would significantly delay the development of markets and products
and could have a material adverse effect on the business, results
of operations and financial condition of the Group.
Failure
to comply with the laws and regulations applicable to its
operations may lead to possible sanctions including the revocation
or imposition of additional conditions on licenses to operate the
Group’s business, the suspension or expulsion from a
particular market or jurisdiction or of its key personnel, and the
imposition of fines and censures. To the extent that there are
changes to the existing laws and regulations or the enactment of
future laws and regulations that affect the sale or offering of the
Group’s products or services in any way, this could have a
material adverse effect on the business, results of operations and
financial condition of the Group.
IM
Cannabis Corp.
Management’s Discussion and Analysis (Canadian dollars, in
thousands)
(ix) Environmental
and Employee Health and Safety Regulations
The
Group’s operations are subject to environmental and
occupational safety laws and regulations in certain jurisdictions,
concerning, among other things, emissions and discharges to water,
air and land, the handling and disposal of hazardous and
nonhazardous materials and wastes, and employee health and safety.
The Group incurs ongoing costs and obligations related to
compliance with environmental and employee health and safety
matters. Any failure to comply or maintain compliance with
environmental and occupational safety laws and regulations may
result in additional costs for corrective measures, penalties or
restrictions on manufacturing operations. In addition, changes in
environmental, employee health and safety or other laws, more
vigorous enforcement thereof or other unanticipated events could
require extensive changes to the Group’s operations or give
rise to material liabilities, which could have a material adverse
effect on the business, results of operations and financial
condition of the Group. This is particularly relevant for Focus,
TJAC, Highland, Sublime and Adjupharm as these entities engage in
cannabis-related operations that may be more prone to environmental
and employee safety issues. Any changes to current laws and
regulations may require substantial investments by the Group in
order to comply such changes. If substantial investments are
required, there may be a material adverse effect on the
Group’s operations, financial condition and operating
results.
(x) Reliance
on License and Permit Renewals
Focus,
Adjupharm, TJAC, Highland and Sublime are dependent on the Focus
License, Adjupharm Licenses, TJAC License and MYM Licenses
(together, the “Key Licenses”), respectively, and the
need to maintain such Key Licenses in good standing. Failure to
comply with the requirements or maintenance of any of the Key
Licenses may have a material adverse effect on the business,
financial condition and operating results of the Group. As of the
date of this MD&A, the Focus License is valid until January 3,
2022, the TJAC Licenses are valid until August 28, 2023, the MYM
Licenses are valid until November 27, 2023 (Highland) and January
31, 2023 (Sublime). The quantities for import and export under the
Adjupharm Licenses are valid until June 18, 2022. Although
management of Focus, Adjupharm, TJAC, Highland and Sublime believe
that they will continue to meet the requirements of the MOH, BfArM
and Health Canada, respectively, for the respective durations of
the Key Licenses, there can be no guarantee that the MOH, BfArM or
Health Canada will extend or renew any of the Key Licenses or, if
any of the Key Licenses are extended or renewed, that they will be
extended or renewed on the same or similar terms.
Should
any of the MOH, BfArM or Health Canada not extend or renew any of
the Key Licenses, or should the Key Licenses be renewed on
different terms or not allow for anticipated capacity increases,
the business, financial condition, results of the operations and
prospects of the Group may be subject to a material adverse
effect.
(xi) Reliance
on Other Business Licenses, Permits and Approvals
In
addition to the Group’s dependence on the Key Licenses
mentioned above, the Group is also dependent on ancillary business
licenses, permits and approvals granted by government authorities
or other third parties in order to operate effectively including,
without limitation, building permits, municipal permits,
third-party licenses, and foreign trade licenses. Should the Group
fail to maintain any of these licenses, permits and approvals, or
should it fail to renew any of such licenses, permits and approvals
on materially similar or more favorable terms, the business,
financial condition and results of the operations of the Group may
be subject to a material adverse effect.
IM
Cannabis Corp.
Management’s Discussion and Analysis (Canadian dollars, in
thousands)
(xii) Reliance
on Focus Facility
The
Focus License is specific to the Focus Facility, which is subject
to the applicable lease agreements (the “Focus Leases”)
and must remain in good standing for Focus to conduct the medical
cannabis activities authorized thereunder. Adverse changes or
developments affecting the Focus Facility, including but not
limited to the failure to maintain all requisite regulatory and
ancillary permits and licenses, the failure to comply with state or
municipal regulations, or a breach of security, could have a
material adverse effect on the Group’s business, financial
condition, results of operations and prospects.
In
addition, any breach of the Focus Leases or any failure to renew
the Focus Leases on materially similar or more favorable terms, may
have a material adverse effect on the Group’s business,
financial condition, results of operations and prospects, and could
also have an impact on Focus’ ability to continue operating
under the Focus License or to renew the Focus License.
The
Focus Facility is subject to state and municipal regulation and
oversight, including the acquisition of all required regulatory and
ancillary permits to conduct operations or undertake any
construction. Any breach of regulatory requirements, security
measures or other facility requirements, including any failure to
comply with recommendations or requirements arising from
inspections by government regulators at all levels, could also have
an impact on Focus’ ability to maintain its lease agreements
and/or keep the Focus Facility in good standing, and to continue
operating under the Focus License or the prospect of renewing the
Focus License.
The
Focus Facility continues to operate with routine maintenance. Focus
will bear much, if not all, of the costs of maintenance and upkeep
of the Focus Facility, including replacement of components over
time. Focus’ operations and the Group’s financial
performance may be adversely affected if Focus is unable to keep up
with maintenance requirements.
In
December 2020, the Construction Committee advised Focus that it was
the subject of the Construction Allegations. Focus’ directors
and officers, including Oren Shuster and Rafael Gabay, received a
summons and have testified before the Construction Committee. In
January 2021, the MOH advised Focus that it had received a
complaint of the same nature as the Construction Allegations (the
“MOH Allegations”). Focus fully cooperated with the
investigations of both the Construction Committee and the MOH. On
July 11, 2021 the Company was informed that the Construction
Committee initiated the Construction Proceedings against Focus,
Focus’ directors and officers, including Mr. Shuster, and
certain land owners. Currently, the Company does not expect a
material impact on the licensing or normal course operations of
Focus due to the Construction Proceedings. The Company, Focus and
Mr. Shuster are cooperating in all respects with the Construction
Proceedings.
Potential
consequences of the Construction Proceedings and/or the MOH
Allegations may include, but are not limited to: (i) criminal
charges against any or all of Focus or Focus’ shareholders
and directors, including Mr. Shuster and Mr. Gabay; (ii) monetary
penalties or fines; (iii) temporary or permanent suspension of the
Focus License; and (iv) other consequences that may limit, in part
or as a whole, Focus’ operations under the Focus License. A
negative outcome to the Construction Proceedings or the MOH
Allegations may have a material adverse effect on the business,
results of operations and financial conditions of the
Group.
IM
Cannabis Corp.
Management’s Discussion and Analysis (Canadian dollars, in
thousands)
(xiii) Reliance
on the TJAC Leases
The
TJAC Licenses are specific to the facilities operated by TJAC in
Canada (the “TJAC Facilities”), which are subject to
the applicable lease agreements (the “TJAC Leases”) and
such licenses must remain in good standing for TJAC to conduct the
cannabis cultivation, processing and sales activities authorized
thereunder. Adverse changes or developments affecting the TJAC
Leases, including but not limited to the failure to maintain all
requisite regulatory and ancillary permits and licenses, the
failure to comply with provincial or municipal regulations, or a
breach of security, could have a material adverse effect on the
Group’s business, financial condition, results of operations
and prospects.
In
addition, any breach of either of the TJAC Leases or any failure to
renew one or both of the TJAC Leases on materially similar or more
favorable terms, may have a material adverse effect on the
Group’s business, financial condition, results of operations
and prospects, and could also have an impact on TJAC’s
ability to continue operating under the TJAC Licenses or to renew
any of the TJAC Licenses.
The
TJAC Facilities are subject to the TJAC Leases and thus, subject to
provincial and municipal regulation and oversight, including the
acquisition of all required regulatory and ancillary permits to
conduct operations or undertake any construction. Any breach of
regulatory requirements, security measures or other facility
requirements, including any failure to comply with recommendations
or requirements arising from inspections by government regulators
at all levels, could also have an impact on TJAC’s ability to
maintain the TJAC Leases and/or keep TJAC Facilities in good
standing, and to continue operating under the TJAC Licenses or the
prospect of renewing one or both TJAC Licenses.
The
TJAC Facilities continue to operate with routine maintenance. TJAC
will bear some of the costs of maintenance and upkeep of the TJAC
Facilities in accordance with the terms of the respective TJAC
Leases, including replacement of components over time. TJAC’s
operations and the Group’s financial performance may be
adversely affected if TJAC is unable to keep up with maintenance
requirements.
(xiv) Reliance
on the MYM Facilities
The MYM
Licenses are specific to the facilities operated by MYM in Canada
by Sublime and Highland (the “MYM Facilities”), of
which the Sublime facility is subject to a lease agreement (the
“Sublime Lease”) and such licenses must remain in good
standing for MYM to conduct the cannabis cultivation, processing
and sales activities authorized thereunder. Adverse changes or
developments affecting the Sublime Lease, including but not limited
to the failure to maintain all requisite regulatory and ancillary
permits and licenses, the failure to comply with provincial or
municipal regulations, or a breach of security, could have a
material adverse effect on the Group’s business, financial
condition, results of operations and prospects.
In
addition, any breach of the Sublime Lease or any failure to renew
the Sublime Lease on materially similar or more favorable terms,
may have a material adverse effect on the Group’s business,
financial condition, results of operations and prospects, and could
also have an impact on MYM’s ability to continue operating
under the MYM Licenses or to renew any of the MYM
Licenses.
IM
Cannabis Corp.
Management’s Discussion and Analysis (Canadian dollars, in
thousands)
The MYM
Facilities are subject to provincial and municipal regulation and
oversight, including the acquisition of all required regulatory and
ancillary permits to conduct operations or undertake any
construction. Any breach of regulatory requirements, security
measures or other facility requirements, including any failure to
comply with recommendations or requirements arising from
inspections by government regulators at all levels, could also have
an impact on MYM’s ability to maintain the Sublime Lease
and/or keep MYM Facilities in good standing, and to continue
operating or the prospect of renewing one or both MYM
Licenses.
The MYM
Facilities continue to operate with routine maintenance. MYM will
bear some of the costs of maintenance and upkeep of the MYM
Facilities in accordance with the terms of the respective Sublime
Lease and ownership of the Highland facility, including replacement
of components over time. MYM’s operations and the
Group’s financial performance may be adversely affected if
MYM is unable to keep up with maintenance
requirements.
(xv) Dependence
on Senior Management
The
success of the Company is dependent upon the contributions of
senior management. The loss of any of these individuals, or an
inability to attract, retain and motivate sufficient members of
qualified senior management personnel could adversely affect its
business. This risk is partially mitigated by the fact that the
senior management team are significant shareholders in the
Company.
(xvi) Competition
in the Industry
There
is potential that the Group will face intense competition from
other companies, some of which can be expected to have more
financial resources, industry, manufacturing and marketing
experience than the Group. Because of the early stage of the
industry in which The Group operates, the Group expects to face
additional competition from new entrants. If the number of licensed
patients of medical cannabis in Israel increases, the demand for
products will increase and the Group expects that competition will
become more intense, as current and future competitors begin to
offer an increasing number of diversified products and pricing
strategies. The same can be said for the Canadian market. As the
cannabis industry continues to develop in Canada, more companies
may enter the recreational cannabis space increasing competition in
an already competitive market.
There
is also the potential that the industry will undergo consolidation,
creating larger companies that may have increased geographic scope.
Increased competition by larger, better-financed competitors with
geographic advantages could materially and adversely affect the
business, financial condition and results of operations of the
Group.
(xvii) Risks
Inherent in the Agricultural Business
The
Group’s business, specifically as it pertains to Adjupharm,
TJAC, Highland, Sublime and the Company’s relationship with
Focus, involves the growing of cannabis products, which are
agricultural products. As such, the business is subject to the
risks inherent in the agricultural business, such as pests, plant
diseases and similar agricultural risks. Although these companies
and their respective third-party cultivators carefully monitor the
growing conditions with trained personnel and applicable equipment,
there can be no assurance that natural elements will not have a
material adverse effect on the production of its products and
results of operations of the aforementioned Group. Any decline in
production by the Group could have a material adverse effect on its
business, operating results or financial condition.
IM
Cannabis Corp.
Management’s Discussion and Analysis (Canadian dollars, in
thousands)
(xviii) Restrictions
on Sales and Marketing
The
industry is in its early development stage and restrictions on
sales and marketing activities imposed by cannabis regulatory
authorities, various medical associations, other governmental or
quasi-governmental bodies or voluntary industry associations in the
jurisdictions in which the Group operates may adversely affect the
Group’s ability to conduct sales and marketing activities and
could have a material adverse effect on the Company’s
business, operating results, financial condition and
prospects.
The
Group’s success depends on its ability to attract and retain
customers. The way cannabis products are packaged, labelled, and
displayed is strictly regulated in the jurisdictions in which the
Group operates. For example, advertising related to consumption of
cannabis is strictly prohibited in Israel. Such prohibitions may
affect the Company’s ability to establish brand presence,
acquire new customers, retain existing customers and maintain a
loyal customer base. This may ultimately have a material adverse
effect on the Group’s business, financial conditions and
operations.
(xix) Publicity
or Consumer Perception
The
Company believes the medical and Canadian recreational cannabis
industry is highly dependent upon consumer perception regarding the
safety, efficiency and quality of the medical cannabis produced.
Consumer perception of the Group’s products can be
significantly influenced by scientific research or findings,
regulatory investigations, litigation, media attention and other
publicity regarding the consumption of medical and recreational
cannabis products.
There
can be no assurance that future scientific research, findings,
regulatory proceedings, litigation, media attention or other
research findings or publicity will be favorable to the medical and
Canadian recreational cannabis market, or any particular
product(s), or consistent with earlier publicity.
Future
research reports, findings, regulatory proceedings, litigation,
media attention or other publicity that are perceived as less
favorable than, or that question, earlier research reports,
findings or publicity could have a material adverse effect on the
demand for products bearing the Company’s brand and the
business, results of operations, financial condition and the
Company’s cash flows. The Company’s dependence upon
consumer perceptions means that adverse scientific research
reports, findings, regulatory proceedings, litigation, media
attention or other publicity, whether or not accurate or with
merit, could have a material adverse effect on the Company, the
demand for products bearing the Company’s brand, and the
business, results of operations, financial condition and cash flows
of the Company.
Further,
adverse publicity reports or other media attention regarding the
safety, efficacy and quality of medical or recreational cannabis in
general, or the Group’s products specifically, or associating
the consumption of medical and recreational cannabis with illness
or other negative effects or events, could have such a material
adverse effect. Such adverse publicity reports or other media
attention could arise even if the adverse effects associated with
such products resulted from consumers’ failure to consume
such products appropriately or as directed.
IM
Cannabis Corp.
Management’s Discussion and Analysis (Canadian dollars, in
thousands)
(xx) Reliance
on Key Business Inputs
The
Group’s business is dependent on a number of key inputs and
their related costs including raw materials and supplies related to
its growing operations as well as electricity, water, and other
utilities. Any significant interruption or negative change in the
availability or economics of the supply chain for key inputs (e.g.
rising energy costs or inflation) could materially impact the
business, financial condition, and operating results of the
Company. Any ability to secure required supplies and services or to
do so on appropriate terms could also have a materially adverse
impact on the business, financial condition, and operating results
of the Company.
(xxi) Sufficiency
of Insurance
The
Group maintains various types of insurance which may include
product liability insurance (see “Potential Product
Liability” below), errors and omission insurance, directors
and officers insurance, trustees’ insurance, property
coverage, and, general commercial insurance. There is no assurance
that claims will not exceed the limits of available coverage, that
any insurer will remain solvent or willing to continue providing
insurance coverage with sufficient limits or at a reasonable cost;
or, that any insurer will not dispute coverage of certain claims
due to ambiguities in the policies. A judgment against any member
of the Group in excess of available coverage could have a material
adverse effect on the Group in terms of damages awarded and the
impact and reputation of the Group.
(xxii) Potential
Product Liability
IMCC
derives a significant portion of its revenues from Focus, TJAC and
MYM, all of which manufacture products designed to be ingested or
inhaled by humans. Focus, TJAC, and MYM products bearing the
Company’s brands face an inherent risk of exposure to product
liability claims, regulatory action and litigation if such products
are alleged to have caused significant loss or injury. In addition,
the manufacture and sale of Focus, TJAC, and MYM products bearing
the Company’s brands involve the risk of injury or loss to
consumers due to tampering by unauthorized third parties, product
contamination, unauthorized use by consumers or other third
parties. Previously unknown adverse reactions resulting from human
consumption of Focus, TJAC, and MYM products bearing the
Company’s brands alone or in combination with other
medications or substances could occur.
The
Group may be subject to various product liability claims,
including, among others, that products bearing the IMC, Focus,
TJAC, or MYM brands caused injury, illness or loss, include
inadequate instructions for use or include inadequate warnings
concerning possible side effects or interactions with other
substances. A product liability claim or regulatory action against
the Group could result in increased costs, could adversely affect
the Group’s reputation with its clients and consumers
generally, and could have a material adverse effect on our results
of operations and financial condition of the Company.
There
can be no assurances that the Group will be able to obtain or
maintain product liability insurance on acceptable terms or with
adequate coverage against potential liabilities. Such insurance is
expensive and may not be available in the future on acceptable
terms, or at all. The inability to obtain sufficient insurance
coverage on reasonable terms or to otherwise protect against
potential product liability claims could prevent or inhibit the
commercialization of products bearing the Company’s
brand.
IM
Cannabis Corp.
Management’s Discussion and Analysis (Canadian dollars, in
thousands)
(xxiii) Potential
General Litigation
The
Group may become party to litigation from time to time in the
ordinary course of business which could adversely affect its
business. Should any litigation in which member of the Group become
involved be determined against such member of the Group, such a
decision could adversely affect the Group’s ability to
continue operating and the market price for the Common Shares and
could use significant resources. Even if the Group is involved in
litigation and wins, litigation can redirect significant Group
resources.
(xxiv) Potential
Product Recalls
Manufacturers
and distributors of products are sometimes subject to the recall or
return of their products for a variety of reasons, including
product defects, such as contamination, unintended harmful side
effects or interactions with other substances, packaging safety and
inadequate or inaccurate labeling disclosure. If products bearing
the Company’s brands are recalled due to an alleged product
defect or for any other reason, the Group could be required to
incur the unexpected expense of the recall and any legal
proceedings that might arise in connection with the
recall.
The
Group may lose a significant amount of sales and may not be able to
replace those sales at an acceptable margin or at all. In addition,
a product recall may require significant management
attention.
Although
the Group has detailed procedures in place for testing finished
products, there can be no assurance that any quality, potency or
contamination problems will be detected in time to avoid unforeseen
product recalls, regulatory action or lawsuits. Additionally, if
one of the Company’s significant brands were subject to
recall, the image of that brand and the Company could be harmed. A
recall for any one of the foregoing reasons could lead to decreased
demand for the Group’s products and could have a material
adverse effect on the results of operations and financial condition
of the Group. Additionally, product recalls may lead to increased
scrutiny of the Group’s operations by BfarM, the MOH and
Health Canada, or other regulatory agencies, requiring further
management attention and potential legal fees and other
expenses.
(xxv) Acquisitions
of the Consolidated Entities
There
is no guarantee that any or all of the acquisitions of the
Consolidated Entities pursuant to the Pharm Yarok Transaction
and/or the Vironna Transaction, as applicable and as further
described in “Overview of
the Company – The Group and Consolidated Entities – The
Consolidated Entities”, and the Panaxia Transaction,
will be completed in the currently proposed form, if at all.
Completion of such acquisitions and/or transactions will require,
among other things, various regulatory approvals, licenses and
permits, including MOH approval. Although the Company believes that
it will obtain the required approvals, licenses and permits, there
is no guarantee that all required approvals, licenses and permits
will be obtained in a timely fashion or at all.
(xxvi) Management
of Growth and Acquisition Integration
The
Company may be subject to growth related risks including capacity
constraints and pressure on its internal systems and controls. The
ability of the Company to manage growth effectively will require it
to continue to implement and improve its operational and financial
systems and to expand, train and manage its employee base. If the
Company is unable to deal with this growth, any negative impact may
have a material adverse effect on the Company’s business,
financial condition, results of operation and
prospects.
IM
Cannabis Corp.
Management’s Discussion and Analysis (Canadian dollars, in
thousands)
In
addition, the realization of the benefits of acquisitions made by
the Company, including the acquisition of Trichome, MYM, certain
operations from Panaxia as part of the Panaxia Transaction, and,
once completed, the Panaxia Transaction, the Pharm Yarok
Transaction and the Vironna Transaction, depend in part on
successfully consolidating functions and integrating and leveraging
operations, procedures and personnel in a timely and efficient
manner as well as the Company’s ability to share knowledge
and realize revenues, synergies and other growth opportunities from
combining the acquired businesses and operations with those of the
Company. The integration of acquired businesses may depend on a
number of factors, including without limitation: (i) the input of
substantial management effort, time and resources; (ii) the
successful incorporation of key personnel from acquired companies
for post-acquisition periods; and (iii) the execution of effective
non-competition agreements with certain employees or ex-employees
of the acquired companies. Any failure in successfully integrating
acquired businesses may result in a material adverse effect on the
Company’s business, financial condition, operating results
and prospects. The risks we face in connection with an acquisition
include:
●
diversion of
management time and focus from operating our business to addressing
acquisition integration challenges;
●
coordination of
research and development and sales and marketing
functions;
●
retention of
employees from the acquired company;
●
cultural challenges
associated with integrating employees from the acquired company
into our organization;
●
integration of the
acquired company's accounting, management information, human
resources, and other administrative systems;
●
the need to
implement or improve controls, procedures, and policies at a
business that prior to the acquisition may have lacked effective
controls, procedures, and policies;
●
potential
write-offs of intangible assets or other assets acquired in
transactions that may have an adverse effect on our operating
results in a given period;
●
liability for
activities of the acquired company before the acquisition,
including patent and trademark infringement claims, violations of
laws, commercial disputes, tax liabilities, and other known and
unknown liabilities; and
●
litigation or other
claims in connection with the acquired company, including claims
from terminated employees, consumers, former stockholders, or other
third parties.
Any
failure of the Company to address these risks or other problems
encountered in connection with any future acquisitions or
investments could impact or prevent the realization of the
anticipated benefits of these acquisitions or investments, cause us
to incur unanticipated liabilities, and harm our business
generally. Future acquisitions could also result in the incurrence
of debt, contingent liabilities, amortization expenses, or the
impairment of goodwill, any of which could harm our financial
condition.
We may
issue additional shares in connection with such transactions, which
would dilute our other shareholders’ interests in us. The
presence of one or more material liabilities of an acquired company
that are unknown to us at the time of acquisition could have a
material adverse effect on our business, results of operations,
prospects and financial condition. A strategic transaction may
result in a significant change in the nature of our business,
operations and strategy. In addition, we may encounter unforeseen
obstacles or costs in implementing a strategic transaction or
integrating any acquired business into our operations.
IM
Cannabis Corp.
Management’s Discussion and Analysis (Canadian dollars, in
thousands)
(xxvii) U.S.
Operations
The
Company and, to its knowledge, its investees, do not currently
engage in any U.S. cannabis-related activities as defined in CSA
Staff Notice 51-352. To date, the Company has caused its investees
to only conduct business and invest in entities in federally-legal
jurisdictions by including appropriate representations, warranties
and covenants in its agreements with investees. However, an
investee may breach such obligations. Any such violation of such
obligation would result in a breach of the applicable agreement
and, accordingly, may have a material adverse effect on the
business, operations and financial condition of
Company.
(xxviii) COVID-19
The
current global uncertainty with respect to the spread of COVID-19,
the rapidly evolving nature of the pandemic and local and
international developments related thereto and its effect on the
broader global economy and capital markets may impact the
Company’s business in the coming months.
The
Company has taken proactive measures throughout the COVID-19
pandemic to protect the health and safety of its employees, to
continue delivering high quality medical cannabis to its patients,
to continue supplying the Canadian recreational cannabis market
with quality products and to maintain its balance
sheet.
While
the precise impact of the COVID-19 outbreak on the Company remains
unknown, rapid spread of COVID-19 and declaration of the outbreak
as a global pandemic has resulted in travel advisories and
restrictions, certain restrictions on business operations, social
distancing precautions and restrictions on group gatherings which
are having direct impacts on businesses in Canada, the State of
Israel, Germany and around the world and could result in additional
precautionary measures that could impact the Company’s
business. The spread of COVID-19 may also have a material adverse
effect on global economic activity and could result in volatility
and disruption to global supply chains and the financial and
capital markets, which could interrupt supplies and other services
from third parties upon which the Company relies, decrease demand
for products, cause staff shortages, reduced customer traffic, and
increased government regulation, all of which may materially and
negatively impact the business, financial condition and results of
operations of the Company.
(xxix) Focus’
Essential Service Designation
In
response to the COVID-19 pandemic, the State of Israel has
periodically implemented mandatory shut-downs of non-essential
businesses to prevent the spread of COVID-19. Focus’ business
has been deemed an “essential service”, permitting it
to continue production. There is no guarantee that further measures
may nevertheless require Focus to shut down or limit its operations
in the State of Israel. Any disruptions to the business and
operations of Focus in the event that Focus were to lose its
designation as an “essential service” in the State of
Israel may materially and negatively impact the business, financial
condition and results of operations of the Company.
IM
Cannabis Corp.
Management’s Discussion and Analysis (Canadian dollars, in
thousands)
(xxx) The
Company’s status as a “foreign private issuer”
under U.S. securities laws
The
Company is a “foreign private issuer”, under applicable
U.S. federal securities laws, and is, therefore, not subject to the
same requirements that are imposed upon U.S. domestic issuers by
the SEC. Under the United States Securities Exchange Act of 1934,
as amended (the “Exchange Act”), the Company is subject
to reporting obligations that, in certain respects, are less
detailed and less frequent than those of U.S. domestic reporting
companies. As a result, the Company does not file the same reports
that a U.S. domestic issuer would file with the SEC, although the
Company is required to file with or furnish to the SEC the
continuous disclosure documents that it is required to file in
Canada under Canadian securities laws. In addition, the
Company’s officers, directors, and principal shareholders are
exempt from the reporting and short-swing profit recovery
provisions of Section 16 of the Exchange Act. Therefore, the
Company’s shareholders may not know on as timely a basis when
the Company’s officers, directors and principal shareholders
purchase or sell Common Shares, as the reporting periods under the
corresponding Canadian insider reporting requirements are
longer.
As a
foreign private issuer, the Company is exempt from the rules and
regulations under the Exchange Act related to the furnishing and
content of proxy statements. The Company is also exempt from
Regulation FD, which prohibits issuers from making selective
disclosures of material non-public information. While the Company
complies with the corresponding requirements relating to proxy
statements and disclosure of material non-public information under
Canadian securities laws, these requirements differ from those
under the Exchange Act and Regulation FD and shareholders should
not expect to receive the same information at the same time as such
information is provided by U.S. domestic companies. In addition,
the Company may not be required under the Exchange Act to file
annual and quarterly reports with the SEC as promptly as U.S.
domestic companies whose securities are registered under the
Exchange Act.
In
addition, as a foreign private issuer, the Company has the option
to follow certain Canadian corporate governance practices, except
to the extent that such laws would be contrary to U.S. securities
laws, and provided that the Company disclose the requirements it is
not following and describe the Canadian practices it follows
instead. The Company may in the future elect to follow home country
practices in Canada with regard to certain corporate governance
matters. As a result, the Company’s shareholders may not have
the same protections afforded to shareholders of U.S. domestic
companies that are subject to all corporate governance
requirements.
(xxxi) The
Company may lose its status as a foreign private issuer under U.S.
securities laws
In
order to maintain its status as a foreign private issuer, a
majority of the Common Shares must be either directly or indirectly
owned by non-residents of the U.S. unless the Company also
satisfies one of the additional requirements necessary to preserve
this status. The Company may in the future lose its foreign private
issuer status if a majority of its Common Shares are held in the
U.S. and if the Company fails to meet the additional requirements
necessary to avoid loss of its foreign private issuer status. The
regulatory and compliance costs under U.S. federal securities laws
as a U.S. domestic issuer may be significantly more than the costs
incurred as a Canadian foreign private issuer eligible to use the
MJDS. If the Company is not a foreign private issuer, it would not
be eligible to use the MJDS or other foreign issuer forms and would
be required to file periodic and current reports and registration
statements on U.S. domestic issuer forms with the SEC, which are
more detailed and extensive than the forms available to a foreign
private issuer. In addition, the Company may lose the ability to
rely upon exemptions from NASDAQ corporate governance requirements
that are available to foreign private issuers.
IM
Cannabis Corp.
Management’s Discussion and Analysis (Canadian dollars, in
thousands)
(xxxii) The
Company’s status as an “emerging growth company”
under U.S. securities laws
The
Company is an “emerging growth company” as defined in
section 3(a) of the Exchange Act (as amended by the JOBS Act,
enacted on April 5, 2012), and the Company will continue to qualify
as an emerging growth company until the earliest to occur of: (a)
the last day of the fiscal year during which the Company has total
annual gross revenues of US$1,070,000,000 (as such amount is
indexed for inflation every five years by the SEC) or more; (b) the
last day of the fiscal year of the Company following the fifth
anniversary of the date of the first sale of common equity
securities of the Company pursuant to an effective registration
statement under the United States Securities Act of 1933, as
amended; (c) the date on which the Company has, during the previous
three year period, issued more than US$1,000,000,000 in
non-convertible debt; and (d) the date on which the Company is
deemed to be a “large accelerated filer”, as defined in
Rule 12b–2 under the Exchange Act. The Company will qualify
as a large accelerated filer (and would cease to be an emerging
growth company) at such time when on the last business day of its
second fiscal quarter of such year the aggregate worldwide market
value of its common equity held by non-affiliates will be
US$700,000,000 or more.
For so
long as the Company remains an emerging growth company, it is
permitted to and intends to rely upon exemptions from certain
disclosure requirements that are applicable to other public
companies that are not emerging growth companies. These exemptions
include not being required to comply with the auditor attestation
requirements of Section 404 of the Sarbanes-Oxley Act. The Company
cannot predict whether investors will find the Common Shares less
attractive because the Company relies upon certain of these
exemptions. If some investors find the Common Shares less
attractive as a result, there may be a less active trading market
for the Common Shares and the Common Share price may be more
volatile. On the other hand, if the Company no longer qualifies as
an emerging growth company, the Company would be required to divert
additional management time and attention from the Company’s
development and other business activities and incur increased legal
and financial costs to comply with the additional associated
reporting requirements, which could negatively impact the
Company’s business, financial condition and results of
operations.
Changes in Accounting Policies including Initial
Adoption
The
Company’s significant accounting policies under IFRS are
contained in the Annual Financial Statements (refer to Note 2 to
the Annual Financial Statements). Certain of these policies involve
critical accounting estimates as they require management to make
particularly subjective or complex judgments, estimates and
assumptions about matters that are inherently uncertain and because
of the likelihood that materially different amounts could be
reported under different conditions or using different
assumptions.
The
following new accounting standards applied or adopted during the
nine months ended September 30, 2021, had impact on the Interim
Financial Statements:
IFRS 3, “Business Combinations”:
In
October 2018, the IASB issued an amendment to the definition of a
“business” in IFRS 3, “Business
Combinations” (“the 2018 Amendment”). The 2018
Amendment is intended to assist entities in determining whether a
transaction should be accounted for as a business combination or as
an acquisition of an asset.
IM
Cannabis Corp.
Management’s Discussion and Analysis (Canadian dollars, in
thousands)
The
2018 Amendment consists of the following:
1.
Clarification that to meet the definition of a business, an
integrated set of activities and assets must include, as a minimum,
an input and a substantive process that together significantly
contribute to the ability to create output.
2.
Removal of the reference to the assessment whether market
participants are capable of acquiring the business and continuing
to operate it and produce outputs by integrating the business with
their own inputs and processes.
3.
Introduction of additional guidance and examples to assist entities
in assessing whether the acquired processes are
substantive.
4.
Narrowing the definitions of “outputs” and
“business” by focusing on goods and services provided
to customers.
5.
Introducing an optional concentration test that permits a
simplified assessment of whether an acquired set of activities and
assets is not a business.
The
2018 Amendment is to be applied prospectively to all business
combinations and asset acquisitions for which the acquisition date
is on or after the beginning of the first annual reporting period
beginning on or after January 1, 2020, with earlier application
permitted. The 2018 Amendment is not expected to have a material
impact on the Company in the current or future reporting
periods.
Amendment to IAS 1, “Presentation of Financial
Statements”:
In
January 2020, the IASB issued an amendment to IAS 1,
“Presentation of Financial Statements” (“the 2020
Amendment”) regarding the criteria for determining the
classification of liabilities as current or
non-current.
The
2020 Amendment includes the following clarifications:
●
What is meant by a
right to defer settlement;
●
That a right to
defer must exist at the end of the reporting period;
●
That classification
is unaffected by the likelihood that an entity will exercise its
deferral right.
●
That only if an
embedded derivative in a convertible liability is itself an equity
instrument would the terms of a liability not impact its
classification.
The
2020 Amendment is effective for annual periods beginning on or
after January 1, 2023 and must be applied
retrospectively.
IM
Cannabis Corp.
Management’s Discussion and Analysis (Canadian dollars, in
thousands)
The
Company is evaluating the possible impact of the 2020 Amendment on
its current loan agreements.
Financial Instruments
Financial
instruments are measured either at fair value or at amortized cost.
The table below lists the valuation methods used to determine fair
value of each financial instrument.
|
Financial Instruments Measured at Fair Value
|
|
Fair Value Method
|
|
|
|
|
|
Derivative
assets1
|
|
Black
& Scholes model (Level 3 category)
|
|
Unlisted
Warrants1
|
|
Black
& Scholes model (Level 3 category)
|
|
Listed
Warrants1
|
|
Market
price (Level 1 category)
|
|
Loans
receivable
|
|
Discounted
Cashflow Method (Level 3 category)
|
|
Financial Instruments Measured at Amortized Cost
|
|
|
|
Cash
and cash equivalents, Trade receivables and other account
receivables
|
|
Carrying
amount (approximates fair value due to short-term
nature)
|
|
Loans
receivable
|
|
Amortized
Cost (effective interest method)
|
|
Trade
Payables, other accounts payable and accrued expenses
|
|
Carrying
amount (approximates fair value due to short-term
nature)
|
Notes:
(1)
Finance expense
(income) include fair value adjustment of Warrants, Investments,
and Derivative assets measured at fair value, for the nine months
ended September 30, 2021 and 2020, amounted to $21,169 and
$(6,048), respectively.
Finance expense
(income) include fair value adjustment of Warrants, Investments,
and Accounts Receivable measured at fair value, for the three
months ended September 30, 2021 and 2020, amounted to $8,120
and $973, respectively.
The
Group has exposure to the following risks from its use of financial
instruments:
Share price risk
The
Group’s investments in unlisted shares are sensitive to the
market price risk arising from uncertainties about the future value
of these investments. The Group manages the price risk through
diversification and by placing limits on individual and total
investment in shares.
IM
Cannabis Corp.
Management’s Discussion and Analysis (Canadian dollars, in
thousands)
The
Company’s board of directors reviews and approves all
decisions related to investments in shares.
At the
reporting date, the Group’s exposure to investments in
unlisted shares measured at fair value was $2,165.
Credit risk
The
maximum credit exposure at September 30, 2021, is the carrying
amount of cash and cash equivalents, accounts receivable and other
current assets. The Group does not have significant credit risk
with respect to customers. All cash and cash equivalents are placed
with major Israeli financial institutions.
Liquidity risk
As at
September 30, 2021, the Group’s financial liabilities with
liquidity risk consist of trade payables and other accounts
payable, which have contractual maturity dates within one year, and
lease liabilities. The Group manages its liquidity risk by
reviewing its capital requirements on an ongoing basis. Based on
the Group’s working capital position as at September 30,
2021, management considers liquidity risk to be low. The table
below summarizes the maturity profile of the Group’s lease
liabilities based on contractual undiscounted payments (including
interest payments):
September
30, 2021:
|
|
|
|
|
|
|
Lease
liabilities
|
$2,791
|
$11,023
|
$12,202
|
$3,224
|
September
30, 2020:
|
|
|
|
|
|
|
Lease
liabilities
|
$172
|
$312
|
$534
|
-
|
The
maturity profile of the Company’s other financial liabilities
with liquidity risk (trade payables, other account payable and
accrued expenses) as of September 30, 2021 and 2020, are less than
one year.
Currency rate risk
As at
September 30, 2021, a portion of the Group’s financial assets
and liabilities held in Euros, Canadian dollars and US dollars
consist of cash and cash equivalents. The Group’s objective
in managing its foreign currency risk is to minimize its net
exposure to foreign currency cash flows by transacting, to the
greatest extent possible, with third parties as applicable. The
Group does not currently use foreign exchange contracts to hedge
its exposure of its foreign currency cash flows, as management has
determined that this risk is not significant at this point of
time.
IM
Cannabis Corp.
Management’s Discussion and Analysis (Canadian dollars, in
thousands)
Procedures and Internal Control over Financial
Reporting
Internal
control over financial reporting is a process designed to provide
reasonable assurance regarding the reliability of financial
reporting and the preparation of financial statements for external
purposes in accordance with applicable IFRS. Internal control over
financial reporting should include those policies and procedures
that establish the following:
●
maintenance of
records in reasonable detail, that accurately and fairly reflect
the transactions and dispositions of assets;
●
reasonable
assurance that transactions are recorded as necessary to permit
preparation of financial statements in accordance with applicable
IFRS;
●
receipts and
expenditures are only being made in accordance with authorizations
of management or the board of directors; and
●
reasonable
assurance regarding prevention or timely detection of unauthorized
acquisition, use or disposition of the Company’s assets that
could have a material effect on the financial
instruments.
The
Company’s management, with the participation of the Chief
Financial Officer, assessed the effectiveness of the
Company’s internal controls over financial reporting and
concluded that as at September 30, 2021, the Company’s
internal control over financial reporting was effective and yet
constantly seek to improve it.
During
the nine months ended September 30, 2021, the Company did not make
any significant changes to its internal controls over financial
reporting that would have materially affected, or reasonably likely
to materially affect, its internal controls over financial
reporting.
Limitations of Disclosure Controls and Procedures and Internal
Control over Financial Reporting
The
Company’s management, including the Chief Executive Officer
and Chief Financial Officer, believe that due to inherent
limitations, any disclosure controls and procedures or internal
control over financial reporting, no matter how well designed and
operated, can provide only reasonable, not absolute, assurance of
achieving the desired control objectives. Further, the design of a
control system must reflect the fact that there are resource
constraints, and the benefits of controls must be considered
relative to their costs. Also, projections of any evaluation of
effectiveness to future periods are subject to the risk that any
design will not succeed in achieving its stated goals under all
potential future conditions. Accordingly, because of the inherent
limitations in a cost- effective control system, misstatements due
to error or fraud may occur and not be detected. Additionally,
management is required to use judgment in evaluating controls and
procedures.
Additional Information
Additional
information relating to the Company, including the Company’s
Annual Information Form, is available on SEDAR at www.sedar.com.
- - - -
- - - - - - - - - - - - - - - - - - - - -