SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
|
REGISTRATION STATEMENT PURSUANT TO SECTION 12(b) OR 12(g) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the fiscal year ended |
|
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the transition period from ____________________ to ____________________ |
|
SHELL COMPANY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
(Exact name of Registrant as specified in its charter)
(Jurisdiction of incorporation or organization)
(Address of principal executive offices)
(Name, Telephone, E-Mail and/or Facsimile number and Address of Company Contact Person)
|
Title of each class
|
Trading Symbol
|
Name of each exchange on which registered
|
||
|
|
|
|
|
Large accelerated filer ☐
|
Accelerated filer ☐
|
|
|
Emerging growth company
|
|
U.S. GAAP ☐
|
|
Other ☐
|
Yes ☐ No
| 5 | ||
| 6 |
|
9 | ||
|
9 | ||
|
9 | ||
|
A. |
Reserved. |
9 |
|
B. |
Capitalization and Indebtedness |
9 |
|
C. |
Reasons for the Offer and Use of Proceeds |
9 |
|
D. |
Risk Factors |
9 |
|
35 | ||
|
A. |
History and Development of the Company |
35 |
|
B. |
Business Overview |
43 |
|
C. |
Organizational Structure |
53 |
|
D. |
Property, Plants and Equipment |
54 |
| 55 | ||
| 55 | ||
|
A. |
Operating Results |
56 |
|
B. |
Liquidity and Capital Resources |
61 |
|
C. |
Research and Development, Patents and Licences, etc. |
62 |
|
D. |
Trend Information |
62 |
|
E. |
Critical Accounting Estimates. |
63 |
| 67 | ||
|
A. |
Directors and Senior Management |
67 |
|
B. |
Compensation |
70 |
|
C. |
Board Practices |
80 |
|
D. |
Employees |
81 |
|
E. |
Share Ownership |
81 |
|
F. |
Disclosure of a registrant’s action to recover erroneously awarded compensation
|
82 |
| 82 | ||
|
A. |
Major Shareholders |
84 |
|
B. |
Related Party Transactions |
85 |
|
C. |
Interests of Experts and Counsel |
85 |
| 85 | ||
|
A. |
Consolidated Statements and Other Financial Information |
85 |
|
B. |
Significant Changes |
87 |
| 87 | ||
|
A. |
Offer and Listing Details |
87 |
|
B. |
Plan of Distribution |
88 |
|
C. |
Markets |
88 |
|
D. |
Selling Shareholders |
88 |
|
E. |
Dilution |
88 |
|
F. |
Expenses of the Issue |
88 |
| 88 | ||
|
A. |
Share Capital |
88 |
|
B. |
Memorandum and Articles of Association |
88 |
|
C. |
Material Contracts |
91 |
|
D. |
Exchange Controls |
91 |
|
E. |
Taxation |
92 |
|
F. |
Dividends and Paying Agents |
98 |
|
G. |
Statement by Experts |
98 |
|
H. |
Documents on Display |
98 |
|
I. |
Subsidiary Information |
98 |
|
J. |
Annual Report to Security Holders |
98 |
| 98 | ||
| 98 |
| 98 | ||
| 98 | ||
| 99 | ||
|
A. |
Disclosure Controls and Procedures |
99 |
|
B. |
Management’s Annual Report on Internal Control Over Financial Reporting
|
99 |
| C. |
Attestation Report of Registered
Public Accounting Firm |
99 |
|
D. |
Changes in Internal Controls Over Financial Reporting |
99 |
| 100 | ||
| 100 | ||
| 100 | ||
| 101 | ||
| 101 | ||
| 101 | ||
| 101 | ||
| 102 | ||
| 102 | ||
| 102 | ||
| 102 | ||
| 103 |
|
Legal Entity |
Jurisdiction |
Relationship with the
Company |
|
I.M.C Holdings Ltd. (“IMC
Holdings”) |
Israel |
Wholly-owned subsidiary |
|
I.M.C. Pharma Ltd. (“IMC
Pharma”) |
Israel |
Wholly-owned subsidiary of IMC Holdings
|
|
I.M.C Farms Israel Ltd. (“IMC
Farms”) |
Israel |
Wholly-owned subsidiary of IMC Holdings
|
|
Focus Medical Herbs Ltd. (“Focus”)
|
Israel |
Private company over which IMC Holdings
exercises “de facto control” under IFRS 10 |
|
R.A. Yarok Pharm Ltd. (“Pharm
Yarok”) |
Israel |
Wholly-owned subsidiary of IMC Holdings
|
|
Rosen High Way Ltd. (“Rosen
High Way”) |
Israel |
Wholly-owned subsidiary of IMC Holdings
|
|
Revoly Trading and Marketing Ltd. dba Vironna
Pharm (“Vironna”) |
Israel |
Subsidiary of IMC Holdings |
|
Oranim Plus Pharm Ltd. (“Oranim
Plus”) |
Israel |
Subsidiary of IMC Holdings |
|
Trichome Financial Corp. (“Trichome”) *
|
Canada |
Wholly-owned subsidiary |
|
Trichome JWC Acquisition Corp. (“TJAC”) *
|
Canada |
Wholly-owned subsidiary of Trichome
|
|
MYM Nutraceuticals Inc. (“MYM”) *
|
Canada |
Wholly-owned subsidiary of Trichome
|
|
Highland Grow Inc. (“Highland”
or “Highland Grow”) *
|
Canada |
Wholly-owned subsidiary of MYM International
Brands Inc. |
|
Adjupharm GmbH (“Adjupharm”)
|
Germany |
Subsidiary of IMC Holdings |
| • |
the Company’s business objectives and milestones and the anticipated timing of execution; |
| • |
the expected performance of the Company’s business and operations; |
| • |
the exportation of the Company’s cannabis products from Israel; |
| • |
the exportation of cannabis products from Canada to Israel and Germany; |
| • |
the Company’s expansion and development of its foreign operations and supply arrangements; |
| • |
the Company’s intentions regarding leveraging its German operational platform and further developing its presence in Europe;
|
| • |
geographic diversification and brand recognition and the growth of the Company’s brands in the respective jurisdictions;
|
| • |
the future impact of the acquisitions of the Israeli Pharmacies and the Panaxia Transaction (as defined below); |
| • |
the expansion of its Israeli sales channels, distribution, delivery and storage capacity, and reach to medical cannabis patients;
|
| • |
the Company’s retail presence, distribution capabilities and data-driven insights; |
| • |
expectations regarding the Company’s revenues, expenses and profits; |
| • |
the Company’s anticipated operating cash requirements and future financing needs; |
| • |
the anticipated Gross Margins, EBITDA and Adjusted EBITDA from the Company’s operations; |
| • |
the expected increase in revenue and margins in its Israeli medical cannabis market activities arising from its acquisitions
|
| • |
statements relating to the Company exiting the Canadian cannabis market to focus on Israel, Germany and European markets; |
| • |
the Company’s ability to achieve profitability in 2023; |
| • |
the results of the restructuring of the Trichome Group under CCAA; |
| • |
cost savings from restructurings in Israel and Germany; |
| • |
the continued listing of the Company’s common shares in the capital of the Company
(the “Common Shares”) following its successful listing on Nasdaq Capital Market (the
“Nasdaq”); |
| • |
expectations related to demand and momentum in the Company’s Israeli operations; |
| • |
expectations in the growth of demand in the medical cannabis industry, including without limitation, in Israel and Germany;
|
| • |
the competitive conditions of the medical and recreational cannabis industry, including ancillary industries such as medical cannabis
operations consulting; |
| • |
the competitive conditions of the industry, including the Company’s ability to maintain or grow its market share; |
| • |
the anticipated legalization and/or decriminalization of adult-use recreational cannabis in Israel and the Company’s business
intentions in the event such legalization and/or decriminalization occurs; |
| • |
the Company’s anticipated obligations to comply with environmental and employee health and safety matters; |
| • |
the effect of new or altered government regulations with respect to the marketing, acquisition, manufacture, management, transportation,
storage, sale and disposal of cannabis and cannabis products; |
| • |
the grant or renewal of licenses or governmental approvals required to conduct activities related to cannabis; |
| • |
the intentions of management of the Company; |
| • |
the Company’s expectations to meet target production capacity; |
| • |
the impacts of future scientific findings regarding the medical and/or recreational cannabis market; |
| • |
the availability of raw materials and supplies at acceptable quantities, qualities and prices; |
| • |
the scope of protection the Company is able to establish and maintain, if any, for intellectual property rights covering its products;
|
| • |
future liquidity and financial capacity; |
| • |
the Company’s plan with respect to any payments of dividends; |
| • |
the Company’s reliance on third party suppliers and partners and its ability to enter into additional supply agreements to
provide sufficient quantities of medical cannabis to fulfil the Company’s obligations; and |
| • |
the Company’s contractual obligations and commitments. |
| • |
the anticipated increase in demand for medical cannabis in the markets in which the Group operates or is contemplating operations;
|
| • |
the anticipated increase in demand for medical and adult-use recreational cannabis in the markets in which the Company operates;
|
| • |
the Company’s satisfaction of international demand for its products; |
| • |
the Company’s ability to implement its growth strategies and leverage synergies of acquisitions; |
| • |
the Company’s ability to reach patients through e-commerce and brick and mortar retail; |
| • |
the development and introduction of new products; |
| • |
the ability to import and the supply of premium and indoor grown cannabis products from third- party suppliers and partners;
|
| • |
the changes and trends in the cannabis industry; |
| • |
the Company’s ability to maintain and renew or obtain required licenses, permits or authorization related to its domestic and
international operations; |
| • |
the Company’s ability to rely on the export of, creation and maintenance of and maintain a consistent supply of imported cannabis
from suppliers and partners; |
| • |
the ability to maintain cost-efficiencies and network of suppliers to maintain purchasing capabilities; |
| • |
the effectiveness of its products for medical cannabis patients; |
| • |
future cannabis pricing and input costs; |
| • |
cannabis production yields of its third-party cultivation suppliers; |
| • |
the Company being able to continue to drive growth from suppliers and partners into Israel, Germany and Europe; |
| • |
the Company’s ability to market its brands and services in Israel, Germany and Europe successfully to its anticipated customers.
|
| • |
the legalization and/or decriminalization of adult-use recreational cannabis and the demand for adult-use recreational cannabis products
in the markets in which the Company operates; and |
| • |
general business risk and liability, including claims or complaints in the normal course of business; |
| • |
any failure of the Company to maintain “de facto” control over Focus Medical in accordance with IFRS 10; |
| • |
regulatory authorities in Israel viewing the Company as the deemed owner of more than 5% of Focus Medical or licensed entities in
contravention of Israeli regulations; |
| • |
limitations on stockholdings of the Company in connection with its direct engagement in the Israeli medical cannabis market;
|
| • |
the ability and/or need to obtain additional financing for continuing operations; |
| • |
the lack of control over the Company’s investees; |
| • |
the risk of defaulting on existing debt; |
| • |
the Company’s ability to continue as a going concern; |
| • |
the ability of the Company to access future financing if needed or on terms acceptable to the Company; |
| • |
the failure of the Company to comply with applicable regulatory requirements in a highly regulated industry; |
| • |
unexpected changes in governmental policies and regulations affecting the production, distribution, manufacture or |
| • |
use of medial cannabis in any jurisdictions in which the Company currently operates or intends to operate; |
| • |
the Company’s ability to continue to meet the listing requirements of the Canadian Security Exchange (“CSE”) and
the NASDAQ; |
| • |
the Israeli government deciding to abandon the decriminalization or legalization of adult-use recreational cannabis; |
| • |
any change in the political environment which would negatively affect the prospect of decriminalization or legalization of adult-use
recreational cannabis in Israel; |
| • |
any unexpected failure of Focus Medical to maintain in good standing or renew its licenses; |
| • |
any adverse outcome of the Construction Proceedings; |
| • |
any unexpected failure of Adjupharm to maintain in good standing or renew any of its Adjupharm Licenses; |
| • |
the Group’s ability to maintain ancillary business licenses, permits and approvals required to operate effectively; |
| • |
the interpretation of Company’s acquisitions of companies or assets by tax authorities or regulatory bodies, including but
not limited to the change of control of licensed entities; |
| • |
the ability of the Group to deliver on their sales commitments or growth objectives; |
| • |
the Group’s reliance on third-party supply agreements and its ability to enter into additional supply agreements to provide
sufficient quantities of medical cannabis to fulfil the Group’s obligations; |
| • |
the Group’s possible exposure to liability, the perceived level of risk related thereto, and the anticipated results of any
litigation or other similar disputes or legal proceedings involving the Group, including but not limited to the Construction Proceedings
and the class action proceedings described herein; |
| • |
the impact of increasing competition; |
| • |
any lack of merger and acquisition opportunities; |
| • |
inconsistent public opinion and perception regarding the use of cannabis; |
| • |
engaging in activities considered illegal under US federal law related to cannabis; |
| • |
political instability and conflict in the Middle East, Eastern Europe and Ukraine; |
| • |
adverse market conditions; |
| • |
unexpected disruptions to the operations and businesses of the Group as a result of the COVID-19 global pandemic or other disease
outbreaks including a resurgence in the cases of COVID-19; |
| • |
the inherent uncertainty of production quantities, qualities and cost estimates and the potential for unexpected costs and expenses;
|
| • |
the Group’s ability to sell its products; |
| • |
currency fluctuations; |
| • |
the risk of defaulting on existing debt; |
| • |
inflationary risks; |
| • |
any change in accounting practices or treatment affecting the consolidation of financial results; |
| • |
the costs of inputs; |
| • |
reliance on management; and |
| • |
the loss of key management and/or employees. |
| 1) |
the Company receiving economic benefits from Focus (and the terms of the Commercial Agreements cannot be changed without the approval
of the Company); |
| 2) |
the Company having the option to purchase the divested 74% interest in Focus held by Oren Shuster, the CEO, director and a promoter
of the Company, and Rafael Gabay, a former consultant director, a former consultant and a promoter of the Company; |
| 3) |
Messrs. Shuster and Gabay each being a director of Focus (while Mr. Shuster concurrently being a director, officer and substantial
shareholder of the Company and Mr. Gabay concurrently being a substantial shareholder of the Company); and |
| 4) |
the Company providing management and support activities to Focus through the Services Agreement. |
| • |
diversion of management time and focus from operating our business to addressing acquisition integration challenges; |
| • |
coordination of research and development and sales and marketing functions; |
| • |
retention of employees from the acquired company; |
| • |
cultural challenges associated with integrating employees from the acquired company into our organization; |
| • |
integration of the acquired company's accounting, management information, human resources, and other administrative systems;
|
| • |
the need to implement or improve controls, procedures, and policies at a business that prior to the acquisition may have lacked effective
controls, procedures, and policies; |
| • |
potential write-offs of intangible assets or other assets acquired in transactions that may have an adverse effect on our operating
results in a given period; |
| • |
liability for activities of the acquired company before the acquisition, including patent and trademark infringement claims, violations
of laws, commercial disputes, tax liabilities, and other known and unknown liabilities; and |
| • |
litigation or other claims in connection with the acquired company, including claims from terminated employees, consumers, former
stockholders, or other third parties. |
Events in the Development of the Business
|
The Top-Shelf Collection – The newest
addition to IMC’s brand portfolio, launched in September 2022 as IMC’s premium product line, offers indoor-grown, high-THC
cannabis flowers with strains such as Lemon Rocket and Diesel Drift. Inspired by the 1970’s cannabis culture in America, the Top-Shelf
Collection targets the growing segment of medical patients who are cannabis culture enthusiasts. |
 |

| The WAGNERS™ brand launched in Israel in Q1 2022, with premium indoor-grown cannabis imported from Canada. The WAGNERS™ brand was the first international premium, indoor-grown brand introduced to the Israel cannabis market, at a competitive price point. The WAGNERS™ brand includes the Dark Helmet, Cherry Jam launched in Q1 2022, and Golden Ghost that was launched in Q4 2022. |  |
|
BLKMKT™, the Company’s second Canadian
brand, was introduced to the Israeli market in Q4 2022. |
 |






|
Revenues from Continuing operations - By Product Type |
||||
|
Financial Year |
Medical Cannabis Products |
Adult-Use Recreational Cannabis Products |
Other Products |
Total |
|
2022 |
$48,384 |
- |
$5,952 |
$54,335 |
|
2021 |
$26,449 |
- |
$7,604 |
$34,053 |
|
2020 |
$14,863 |
- |
$1,027 |
$15,890 |
| • |
Cannabi-s flos (3028), |
| • |
Cannabis extractum siccum (3068), |
| • |
Cannabis extractum spissum (3069). |
| • |
The IP Agreement and the Services Agreement (collectively, the “Commercial Agreements”),
whereby IMC Holdings derives economic benefit from Focus and whereby Focus (i) uses the IMC brand on an exclusive basis for the sale of
cannabis products; and (ii) engages IMC Holdings to provide certain management and consulting services. As a result of the Company’s
commercial relationship with Focus, it is dependent on Focus maintaining the Focus License, the Focus Lease Agreement and the Focus Facility
in good standing, as well as any ancillary licenses required to carry on its operations in the Israeli medical cannabis industry.
|
| • |
Supply agreements with third party cannabis cultivators and suppliers to meet the Israeli market’s demand for the Company’s
products. |
| • |
Purchase orders received from time to time for the sale of the Company’s products to pharmacies or distributors, either in
association with Focus or through the Company’s direct trading house operations. |
| • |
Ongoing retail purchases of the Company’s products sold at the Israeli Pharmacies by Israeli medical cannabis patients.
|
|
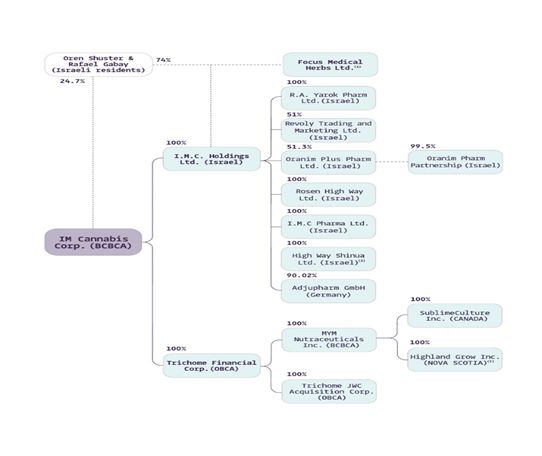
Legal Entity |
Jurisdiction |
Relationship with the Company |
|
I.M.C Holdings Ltd. (“IMC Holdings”)
|
Israel |
Wholly-owned subsidiary |
|
I.M.C. Pharma Ltd. (“IMC Pharma”)
|
Israel |
Wholly-owned subsidiary of IMC Holdings |
|
I.M.C Farms Israel Ltd. (“IMC Farms”)
|
Israel |
Wholly-owned subsidiary of IMC Holdings |
|
Focus Medical Herbs Ltd. (“Focus”)
|
Israel |
Private company over which IMC Holdings exercises “de facto control” under
IFRS 10 |
|
R.A. Yarok Pharm Ltd. (“Pharm Yarok”)
|
Israel |
Wholly-owned subsidiary of IMC Holdings |
|
Rosen High Way Ltd. (“Rosen High Way”)
|
Israel |
Wholly-owned subsidiary of IMC Holdings |
|
Revoly Trading and Marketing Ltd. dba Vironna Pharm (“Vironna”)
|
Israel |
Subsidiary of IMC Holdings |
|
Oranim Plus Pharm Ltd. (“Oranim Plus”)
|
Israel |
Subsidiary of IMC Holdings |
|
Trichome Financial Corp. (“Trichome”) *
|
Canada |
Wholly-owned subsidiary |
|
Trichome JWC Acquisition Corp. (“TJAC”) *
|
Canada |
Wholly-owned subsidiary of Trichome |
|
MYM Nutraceuticals Inc. (“MYM”) *
|
Canada |
Wholly-owned subsidiary of Trichome |
|
Highland Grow Inc. (“Highland”
or “Highland Grow”) *
|
Canada |
Wholly-owned subsidiary of MYM International Brands Inc. |
|
Adjupharm GmbH (“Adjupharm”)
|
Germany |
Subsidiary of IMC Holdings |
|
For the year ended December
31 |
||||||||||||
|
2022 |
2021 |
2020 |
||||||||||
|
Net
Revenues |
$ |
54,335 |
$ |
34,053 |
$ |
15,890 |
||||||
|
Gross
profit before fair value impacts in cost of sales |
$ |
11,291 |
$ |
8,595 |
$ |
8,809 |
||||||
|
Gross
margin before fair value impacts in cost of sales (%) |
21 |
% |
25 |
% |
55 |
% | ||||||
|
Operating
Loss |
$ |
(30,791 |
) |
$ |
(23,035 |
) |
$ |
(8,245 |
) | |||
|
Net loss |
$ |
(24,922 |
) |
$ |
(664 |
) |
$ |
(28,734 |
) | |||
|
Loss
per share attributable to equity holders of the Company – Basic (in CAD) |
$ |
(3.13 |
) |
$ |
0.02 |
$ |
(0.19 |
) | ||||
|
Loss
per share attributable to equity holders of the Company - Diluted (in CAD) |
$ |
(3.81 |
) |
$ |
(3.62 |
) |
$ |
(0.19 |
) | |||
|
Israel |
Germany |
Adjustments |
Total |
|||||||||||||||||||||||||||||
|
2022 |
2021 |
2022 |
2021 |
2022 |
2021 |
2022 |
2021 |
|||||||||||||||||||||||||
|
Revenues |
$ |
50,500 |
$ |
25,431 |
$ |
3,835 |
$ |
8,622 |
$ |
- |
$ |
- |
$ |
54,335 |
$ |
34,053 |
||||||||||||||||
|
Segment income (loss) |
$ |
(23,606 |
) |
$ |
(10,653 |
) |
$ |
(3,225 |
) |
$ |
(5,142 |
) |
$ |
- |
$ |
- |
$ |
(26,831 |
) |
$ |
(15,795 |
) | ||||||||||
|
Unallocated corporate expenses |
$ |
- |
$ |
- |
$ |
- |
$ |
- |
$ |
(3,960 |
) |
$ |
(7,240 |
) |
$ |
(3,960 |
) |
$ |
(7,240 |
) | ||||||||||||
|
Total operating (loss) income |
$ |
(23,606 |
) |
$ |
(10,653 |
) |
$ |
(3,225 |
) |
$ |
(5,142 |
) |
$ |
(3,960 |
) |
$ |
(7,240 |
) |
$ |
(30,791 |
) |
$ |
(23,035 |
) | ||||||||
|
Depreciation, amortization & impairment |
$ |
6,747 |
$ |
1,424 |
$ |
200 |
$ |
701 |
$ |
- |
$ |
- |
$ |
6,947 |
$ |
2,125 |
||||||||||||||||
|
Israel |
Germany |
Adjustments |
Total |
|||||||||||||||||||||||||||||
|
2022 |
2021 |
2022 |
2021 |
2022 |
2021 |
2022 |
2021 |
|||||||||||||||||||||||||
|
Revenues |
$ |
13,136 |
$ |
8,472 |
$ |
1,325 |
$ |
1,440 |
$ |
- |
$ |
- |
$ |
14,461 |
$ |
9,912 |
||||||||||||||||
|
Segment income (loss) |
$ |
(10,280 |
) |
$ |
(4,425 |
) |
$ |
(517 |
) |
$ |
(2,738 |
) |
$ |
- |
$ |
- |
$ |
(10,797 |
) |
$ |
(7,163 |
) | ||||||||||
|
Unallocated corporate income (expenses) |
$ |
- |
$ |
- |
$ |
- |
$ |
- |
$ |
90 |
$ |
(1,578 |
) |
$ |
90 |
$ |
(1,578 |
) | ||||||||||||||
|
Total operating (loss) income |
$ |
(10,280 |
) |
$ |
(4,425 |
) |
$ |
(517 |
) |
$ |
(2,738 |
) |
$ |
90 |
$ |
(1,578 |
) |
$ |
(10,707 |
) |
$ |
(8,741 |
) | |||||||||
|
Depreciation, amortization & impairment |
$ |
4,957 |
$ |
(1,217 |
) |
$ |
48 |
$ |
635 |
$ |
- |
$ |
- |
$ |
5,005 |
$ |
(582 |
) | ||||||||||||||
| ● |
Revenues from continuing operations for the year ended December 31, 2022 and 2021 were $54,335 and $34,053, respectively, representing
an increase of $20,282 or 60%. Revenues for the three months ended December 31, 2022, and 2021 were $14,461 and $9,912, respectively,
representing an increase of $4,549 or 46%. The increase in revenues is primarily attributed to the increase in the quantity of medical
cannabis products sold, as well as from the higher average selling price per gram the Company realized from its portfolio of premium branded
cannabis products in Israel. Additional increases were derived from the Company’s organic growth and related synergies in the areas
where it operates.
|
| ● |
Revenues from the Israeli operation were attributed to the sale of medical cannabis through the Company’s agreement with Focus
Medical and the revenues from the Israeli Pharmacies the Company owns, mostly from cannabis products.
|
| ● |
In Germany, Company revenues were attributed to the sale of medical cannabis through Adjupharm. |
| 1. |
Selling price per gram - calculated as the weighted average historical selling price for all strains of cannabis sold by the Group,
which is expected to approximate future selling prices. |
| 2. |
Post-harvest costs - calculated as the cost per gram of harvested cannabis to complete the sale of cannabis plants post-harvest,
consisting of the cost of direct and indirect materials, depreciation and labor as well as labelling and packaging costs. |
| 3. |
Attrition rate - represents the weighted average percentage of biological assets which are expected to fail to mature into cannabis
plants that can be harvested. |
| 4. |
Average yield per plant - represents the expected number of grams of finished cannabis inventory which are expected to be obtained
from each harvested cannabis plant. |
| 5. |
Stage of growth - represents the weighted average number of weeks out of the average weeks growing cycle that biological assets have
reached as of the measurement date. The growing cycle is approximately 12 weeks. |
|
10% change as of |
||||||||||||||||
|
December 31, 2022 |
December 31, 2021 |
December 31, 2022 |
December 31, 2021 |
|||||||||||||
|
In CAD |
In Thousands of CAD |
|||||||||||||||
|
Average selling price per gram of dried
cannabis |
$ |
3.21 |
$ |
3.64 |
$ |
60 |
$ |
296 |
||||||||
|
Average post-harvest costs per gram of
dried cannabis |
$ |
0.75 |
$ |
1.16 |
$ |
17 |
$ |
140 |
||||||||
|
Attrition rate |
51 |
% |
27 |
% |
44 |
% |
100 |
% | ||||||||
|
Average yield per plant (in grams)
|
38 |
47 |
42 |
228 |
||||||||||||
|
Average stage of growth |
82 |
% |
47 |
% |
39 |
% |
212 |
% | ||||||||
| ● |
production costs (current period costs that are directly attributable to the cannabis growing and harvesting process); |
| ● |
materials and finished goods purchase costs; |
| ● |
a fair value adjustment on sale of inventory (the change in fair value associated with biological assets that were transferred to
inventory upon harvest); and |
| ● |
a fair value adjustment on growth of biological assets (the estimated fair value less cost to sell of biological assets as at the
reporting date). |
| ● |
Through the Trichome Transaction, the Company recognized goodwill of approximately $67,269 and intangible assets, primarily attributed
to the cultivation license, worth approximately $6,458 (based on a preliminary purchase price allocation). The goodwill arising on acquisition
was attributed to the expected benefits from the synergies of the combination of the activities of the Company and Trichome, as well as
value attributed to the assembled workforce, which was included in goodwill. The goodwill recognized was not expected to be deductible
for income tax purposes. The Canadian Restructuring and commencement of an exit from the Canadian market, which was announced on November
7, 2022, resulted in indicators of impairment under IAS 36. These indicators of impairment led to an impairment analysis, in which it
was concluded that a write-down was required. In Q3 2022, an impairment loss of $67,171 was recorded for the goodwill initially recognized
through the Trichome Transaction. |
| ● |
The Company recognized the fair value of the assets acquired and liabilities assumed in the business combination according to a provisional
measurement. The purchase consideration and the fair value of the acquired assets and liabilities may be adjusted within 12 months from
the acquisition date. At the date of final measurement, adjustments are generally made by restating comparative information previously
determined provisionally. As of the date of the Annual Financial Statements, a final valuation for the fair value of the identifiable
assets acquired and liabilities assumed by an external valuation specialist had been obtained. |
| ● |
On July 9, 2021, the Company completed the MYM Transaction. As a result, the Company recognized goodwill of approximately $39,932
and intangible assets consisting of brand name and customer relationships worth approximately $17,200 (based on a preliminary purchase
price allocation study). The goodwill arising on acquisition was attributed to the expected benefits from the synergies of the combination
of the activities of the Company and MYM, as well as value attributed to the assembled workforce, which was included in goodwill. The
goodwill recognized was not expected to be deductible for income tax purposes. As part of the closure of the Sublime Transaction the Company
recorded an impairment loss for the intangible assets in the amount of $1,581. |
| ● |
The Company recognized the fair value of the assets acquired and liabilities assumed in the business combination according to a provisional
measurement. The purchase consideration and the fair value of the acquired assets and liabilities may be adjusted within 12 months from
the acquisition date. At the date of final measurement, adjustments are generally made by restating comparative information previously
determined provisionally. As of the date of the Annual Financial Statements, a final valuation for the fair value of the identifiable
assets acquired and liabilities assumed by an external valuation specialist had been obtained. |
| a. |
Amendment to IAS 1, "Presentation of Financial Statements": |
| - |
Only covenants with which an entity must comply on or before the reporting date will affect a liability's classification as current
or non-current. |
| - |
An entity should provide disclosure when a liability arising from a loan agreement is classified as non-current and the entity's
right to defer settlement is contingent on compliance with future covenants within twelve months from the reporting date. This disclosure
is required to include information about the covenants and the related liabilities. The disclosures must include information about the
nature of the future covenants and when compliance is applicable, as well as the carrying amount of the related liabilities. The purpose
of this information is to allow users to understand the nature of the future covenants and to assess the risk that a liability classified
as non-current could become repayable within twelve months. Furthermore, if facts and circumstances indicate that an entity may have difficulty
in complying with such covenants, those facts and circumstances should be disclosed. |
| b. |
Amendment to IAS 8, "Accounting Policies, Changes to Accounting Estimates and Errors": |
| c. |
Amendment to IAS 12, "Income Taxes": |
| d. |
Amendment to IAS 1, "Disclosure of Accounting Policies": |
|
Nominee Name and Place of Residence |
Position with IM Cannabis Corp. |
Present and Principal Occupation, Business or Employment for Previous
5 years |
Became Director/Executive Officer |
Number of Common Shares beneficially owned, controlled or directed
|
|
Oren Shuster(3)
Ra’anana, Israel |
Chief Executive Officer & Director |
CEO of the Company since October 2019; Founder and director of I.M.C. Holdings Ltd.
since 2018; Founder and director of Focus Medical Herbs Ltd. since 2010; Founder of Ewave Group Ltd. |
October 11, 2019 |
1,872, 870 (4)
|
|
Marc Lustig West Vancouver, British Columbia, Canada |
Executive Chairman and Director |
Executive Chairman of the Company since December 2020; Director of Pharmacielo Ltd.
since November 2020; Director of Cresco Labs Inc. since June 2020; Director of Trichome Financial Corp. since October 2019; Founder, Chairman
and Chief Executive Officer of CannaRoyalty Corp. (dba Origin House) from 2016 to 2020. Director of Briacell Therapeutics Corp., a Nasdaq
listed biotechnology company since September 2021. |
October 11, 2019 |
338,144 |
|
Moti Marcus(1)(2)(3) Tel Aviv, Israel |
Director |
Chief Executive Officer of Packer Quality Metals Ltd. since 2019; Chief Financial
Officer and deputy Chief Executive Officer of S. Cohen Metal Works Ltd. from 2013 and 2018. |
September 12, 2022 |
Nil |
|
Einat Zakariya(1)(2)(3) Herzliya, Israel |
Director |
Chief Executive Officer and Partner of Liv Residence Ltd., a subsidiary of Ewave Holdings
Ltd.; Chief Executive Officer and Partner of Ewave Nadlan International Investments Ltd., since 2018; Chief Executive Officer and Partner
of The Promised Land, a subsidiary of Ewave Nadlan International Investments Ltd., from 2014 to 2018. |
September 12, 2022 |
61,200 |
|
Brian Schinderle(1)(2) Illinois, USA |
Director |
Founder and Manager of Solidum Capital since 2017; Executive Vice President of Finance
of GHG Management (dba Grassroots Cannabis) from 2018 to 2020; Portfolio Manager of Balyasny Asset Management from 2009 to 2017. |
February 22, 2021 |
Nil |
|
Shai Shemesh |
Chief Financial Officer(6)
|
Group CFO at IMC since 2019; CFO at IVM Minrav Sadyt, 2011-2019.
|
October 11, 2019 |
21,190 |
|
Rinat Efrima(5) |
Chief Executive Officer of Subsidiary, IMC Holdings(7)
|
Managing Director Israel and Global Chief Marketing Officer at Caesarstone Ltd. Since
June 2019 to February 2022; Sector General Manager for Europe, Middle East and Africa at Kimberly-Clark Corporation since September 2015
to May 2019. |
March 1, 2022 |
15,000 |
|
Yael Harrosh |
Chief Legal and Operations Officer |
Group’s CLO since 2019 and COO since 2022; Deputy CEO and legal counsel at Promarket
Group, 2016-2018. Prior to that, associate at top law firms in Israel, |
October 11, 2019 |
Nil |
|
Richard Balla |
Chief Executive Officer of Subsidiary |
Managing Director of Adjupharm, Head of Market and Product Development at ACA Müller
Pharma AG |
October 11, 2019 |
Nil |
|
Itay Vago(6) |
Incoming Chief Financial Officer(6)
|
Finance Director of IMC Holdings Ltd., the Company’s Israeli subsidiary (“IMC
Holdings”), 2022-2023. Senior Finance Controller at AstraZeneca Ltd, 2018-2022; APAC CFO at Telit Communication PLC, 2014-2018.
|
(6)March
8, 2023 |
Nil |
|
Eyal Fisher(7) |
Incoming General Manager Subsidiary, IMC Holdings(7)
|
Deputy CEO and Sales & Trade Director of IMC Holdings since 2021; Vice President
Sales and Division Manager at Pandora Jewellery, 2016-2020. |
(7)March
8, 2023 |
Nil |
| (1) |
Member of the Audit Committee. |
| (2) |
Member of Compensation Committee. |
| (3) |
Member of the Governance and Nomination Committee. |
| (4) |
1,872,717 Common Shares are held by Oren Shuster directly and 153 Common Shares are held indirectly through Ewave Group Ltd., a privately-held
entity of which Mr. Shuster owns and controls 50% of the outstanding voting. |
| (5) |
Ms. Efrima is the Chief Executive Officer of IMC Holdings Ltd. |
| (6) |
As per the organizational changed announced on March 8, 2023, Mr. Vago will replace Mr. Shemesh as CFO of the Company pursuant to
a structured transition period;
|
| (7) |
As per the organizational changed announced on March 8, 2023, Mr. Fisher will replace Ms. Efrima as General Manager of IMC Holdings
pursuant to a structured transition period; |
|
Board Diversity Matrix (As of March 29, 2023) | ||||
|
Country of Principal Executive Offices: |
Israel | |||
|
Foreign Private Issuer |
Yes | |||
|
Disclosure Prohibited under Home Country Law |
No | |||
|
Total Number of Directors |
5 | |||
|
|
Male |
Female |
Non-Binary |
Did Not Disclose Gender |
|
Part I: Gender Identity |
| |||
|
Directors |
4 |
1 |
– |
– |
|
Part II: Demographic Background |
| |||
|
Under-represented person in Home Country |
0 | |||
|
LGBTQ+ |
0 | |||
| 1. |
base salary; |
| 2. |
cash bonuses; and/or |
| 3. |
long-term incentives. |
| 1. |
Base Salary |
| 2. |
Cash Bonuses |
| 3. |
Long Term Incentives |
| (a) |
the maximum number of RSUs available for grant to any one person under the RSU Plan and any other Securities Based Compensation Arrangements
of the Company in a 12 month period is 5% of the total number of Common Shares then outstanding on a non-diluted basis; and |
| (b) |
the maximum number of Common Shares issuable to insiders of the Company (as a group) under the RSU Plan, together with any other
Common Shares issuable under any other Securities Based Compensation Arrangements, shall not exceed at any time or within any 12 month
period, 10% of the issued and outstanding Common Shares on a non-diluted basis at the time of grant. |
| (a) |
increase the number of Common Shares which may be issued pursuant to the RSU Plan, other than by virtue of a change in Common Shares,
whether by reason of a stock dividend, consolidation, subdivision or reclassification which adjustment may be made by the Board or Compensation
Committee for the number of Common Shares available under the RSU Plan and the number of Common Shares subject to RSUs; |
| (b) |
amend the definition of “Participant” under the RSU Plan which would have the potential of narrowing, broadening or increasing
insider participation; |
| (c) |
amendments to cancel and reissue RSUs; |
| (d) |
amendments to the list of amendments to the RSU Plan or RSUs requiring requisite regulatory and shareholder approval and those subject
to requisite regulatory approval (where required) but not subject to shareholder approval; |
| (e) |
amendments that extend the term of an RSU; |
| (f) |
amendments to the participation limits including: the maximum number of shares issuable under the RSU Plan, limitations on grants
of RSUs to any one person in a 12-month period, grants within a one year period to insiders, and the number of shares issuable to a person
providing investor relations activities in any 12-month period; and |
| (g) |
amendments to the RSU Plan that would permit RSUs, or any other right or interest of a RSU Participant under the RSU Plan, to be
assigned or transferred, other than for normal estate settlement purposes. |
| (a) |
amendments of a housekeeping nature; |
| (b) |
amendments to the vesting provisions of a RSU or the RSU Plan; |
| (c) |
amendments to the definitions, other than such definitions noted above; |
| (d) |
amendments to reflect changes to applicable securities laws; and |
| (e) |
amendments to ensure that the RSUs granted under the RSU Plan will comply with any provisions respecting income tax and other laws
in force in any country or jurisdiction of which a RSU Participant to whom a RSU has been granted may from time to time be a resident,
citizen or otherwise subject to tax therein. |
|
Name and Principal Position
|
Year(1)
|
Salary
($)
|
Share-Based Awards
($)
|
Option-Based Awards
($) (7)
|
Non-Equity Incentive
Plan Compensation
($) |
All Other
Compensation ($) |
Total
Compensation ($) | |
|
Annual Incentive Plans
|
Long-Term Incentive
Plans | |||||||
|
Oren Shuster(1)
CEO and Director
|
2022 |
506,244 |
Nil |
1,110,057 |
Nil |
Nil |
Nil |
1,616,301 |
|
2021 |
515,731 |
Nil |
1,388,455 |
121,000 |
Nil |
Nil |
2,025,186 | |
|
2020 |
424,492 |
Nil |
202,743 |
110,000 |
Nil |
4,577 |
741,812 | |
|
Shai Shemesh(2)
CFO |
2022 |
321,950 |
Nil |
307,636 |
Nil |
Nil |
Nil |
629,586 |
|
2021 |
300,607 |
Nil |
408,653 |
82,500 |
Nil |
Nil |
791,760 | |
|
2020 |
249,960 |
Nil |
112,390 |
75,000 |
Nil |
Nil |
437,350 | |
|
Marc Lustig
Executive Chairman and
Director(3) (6)
|
2022 |
282,480 |
558,538
|
50,089 |
Nil |
Nil |
Nil |
891,107 |
|
2021 |
264,000 |
1,286,498 |
329,846 |
Nil |
Nil |
Nil |
1,880,344 | |
|
2020 |
90,000 |
Nil |
1,059,085 |
Nil |
Nil |
500,000 |
1,649,085 | |
|
Michael Ruscetta
Chief Executive Officer
of a subsidiary(4) |
2022 |
128,497 |
Nil |
464,310 |
Nil |
Nil |
Nil |
597,807 |
|
2021 |
201,250 |
Nil |
675,719 |
175,000 |
Nil |
Nil |
1,051,969 | |
|
2020 |
Nil |
Nil |
Nil |
Nil |
Nil |
Nil |
Nil | |
|
Howard Steinberg
Chief Executive Officer
of a subsidiary(5) |
2022 |
704,688 |
Nil |
464,310 |
Nil |
Nil |
Nil |
1,168,998 |
|
2021 |
480,000 |
Nil |
675,719 |
400,000 |
Nil |
Nil |
1,555,719 | |
|
2020 |
Nil |
Nil |
Nil |
Nil |
Nil |
Nil |
Nil | |
| (1) |
Mr. Shuster was appointed CEO and director of the Company on October 11, 2019. Mr. Shuster does not earn consideration for his role
as a director of the Company. |
| (2) |
Mr. Shemesh was appointed CFO of the Company on October 11, 2019. |
| (3) |
Mr. Lustig was appointed Executive Chairman of the Company on December 29, 2020. Mr. Lustig does not earn consideration for his role
as a director of the Company. |
| (4) |
Mr. Ruscetta is the Chief Executive Officer of Trichome. |
| (5) |
Mr. Steinberg is the Chief Executive Officer of TJAC and MYM. |
| (6) |
On September 21, 2021 the Company granted Mr. Lustig 550,000 RSUs. |
| (7) |
The Company used the Black-Scholes pricing model as the methodology to calculate the grant date fair value, and relied on the following
the key assumptions and estimates for each calculation under the following assumptions: (i) risk free interest rate of 0.42% to 1.97%
(ii) expected dividend yield of 0%; (iii) expected volatility of 76.28% to 82.31%; and (iv) a term of 5 to 10 years. The Black-Scholes
pricing model was used to estimate the fair value as it is the most accepted methodology. |
|
Option-based
Awards |
Share-based
Awards | ||||||
|
Name
|
Number
of securities underlying unexercised options(1) (2)
(#)
|
Option
exercise price
($)(3)
|
Option
expiration date |
Value
of unexercised
in-the-money options(3) ($)
|
Number
of shares or units of shares that have not vested
(#)
|
Market
or payout value of share-based awards that have not vested(4)
($)
|
Market or payout value
of vested share-based awards not paid out or distributed
($) |
|
Oren Shuster
CEO and Director
|
6,250
75,000 50,000 |
40
58.7
16 |
June 9, 2025
May 19, 2026
January 4, 2029 |
Nil Nil Nil |
Nil |
Nil |
Nil |
|
Shai Shemesh
CFO |
3,750
20,165 6,250 |
40
58.7
16 |
June 9, 2025
May 19, 2026
April 7, 2029 |
Nil Nil Nil |
Nil |
Nil |
Nil |
|
Yael Harrosh
Chief Legal and Operations
Officer |
3,750
18,707 5,000 |
40
58.7
16 |
June 9, 2025
May 19, 2026
January 4, 2029 |
Nil Nil Nil |
Nil |
Nil |
Nil |
|
Marc Lustig(1)
Executive Chairman
and Director |
67,500 |
16 |
September 11, 2029 |
Nil |
13,757 |
17,884 |
53,616 |
|
Rinat Efrima
Chief Executive Officer
of a subsidiary |
5,000 |
27.3 |
April 4, 2027 |
Nil |
Nil |
Nil |
Nil |
|
Richard Balla
Chief Executive Officer
of a subsidiary |
3,750 |
16 |
July 7, 2029 |
Nil |
Nil |
Nil |
Nil |
|
Michael Ruscetta
Chief Executive Officer
of a subsidiary |
23,250 |
100.2 |
March 18, 2026 |
Nil |
Nil |
Nil |
Nil |
|
Howard Steinberg
Chief Executive Officer
of a subsidiary |
23,250 |
100.2 |
March 18, 2026 |
Nil |
Nil |
Nil |
Nil |
| (1) |
Mr. Lustig was appointed as board member on October 11, 2019, and as Executive Chairman on December 29, 2020. |
| (2) |
Each Option entitles the holder to purchase one Common Share. |
| (3) |
On February 12, 2021, the Company completed a consolidation of its Common Shares on a 4:1 basis. The figures reported in this table
are presented on a 4:1 post-consolidation basis. |
| (4) |
Calculated using the closing market price of the Common Shares on the CSE on December 31, 2022 of $1.3 and subtracting the exercise
price of in-the-money Options, including unvested. These Options have not been, and may never be, exercised and actual gains, if any,
on exercise will depend on the value of the Common Shares on the date of exercise. |
| (5) |
Calculated using the closing market price of the Common Shares on the CSE on December 31, 2022 of $1.3. |
|
Name |
Option-based awards – Value vested during the year
($) |
Share-based awards – Value vested during the year ($)
|
Non-equity incentive plan compensation – Value earned during
the year ($) |
|
Oren Shuster
CEO |
1,410,332 |
Nil |
Nil |
|
Shai Shemesh
CFO |
429,862 |
Nil |
Nil |
|
Yael Harrosh
Chief Legal and Operations
Officer |
302,668 |
Nil |
Nil |
|
Marc Lustig
Executive Chairman
and Director |
536,117 |
641,982 |
Nil |
|
Rinat Efrima
Chief Executive Officer
of a subsidiary |
Nil |
Nil |
Nil |
|
Richard Balla
Chief Executive Officer
of a subsidiary |
37 |
Nil |
Nil |
|
Michael Ruscetta
Chief Executive Officer
of a subsidiary |
732,792 |
Nil |
Nil |
|
Howard Steinberg
Chief Executive Officer
of a subsidiary |
732,792 |
Nil |
Nil |
|
Name |
Fees earned ($)
|
Share-based awards
($) |
Option-based awards
($)
(5)
|
Non-equity incentive
plan compensation ($) |
Pension value ($)
|
All other compensation
($) |
Total ($) |
|
Vivian Bercovici Director(1)
|
58,530 |
Nil |
75,918 |
Nil |
Nil |
Nil |
134,448 |
|
Haleli Barath(2)
Director |
59,560 |
Nil |
205,764 |
Nil |
Nil |
Nil |
265,324 |
|
Brian Schinderle
Director |
86,797 |
Nil |
205,764 |
Nil |
Nil |
Nil |
292,561 |
|
Moti Marcus(3) Director
|
24,722 |
Nil |
7,114 |
Nil |
Nil |
Nil |
31,836 |
|
Einat Zakariya (4) Director
|
24,621 |
Nil |
7,114 |
Nil |
Nil |
Nil |
31,735 |
| (1) |
Ms. Bercovici resigned on September 13, 2022. |
| (2) |
Ms. Barath resigned on September 13, 2022. |
| (3) |
Mr. Marcus was appointed on September 13, 2022. |
| (4) |
Ms. Zakariya appointed on September 13, 2022 |
| (5) |
The Company used the Black-Scholes pricing model as the methodology to calculate the grant date fair value, and relied on the following
the key assumptions and estimates for each calculation under the following assumptions: (i) risk free interest rate of 0.42% to 3.03%
(ii) expected dividend yield of 0%; (iii) expected volatility of 78.7% to 82.01%; and (iv) a term of 5 to 10 years. The Black-Scholes
pricing model was used to estimate the fair value as it is the most accepted methodology. |
|
Option-based
Awards |
Share-based
Awards | ||||||
|
Name
|
Number
of securities underlying unexercised options(1) (2)
(#)
|
Option
exercise price
($)(2)
|
Option
expiration date |
Value
of unexercised
in-the-money options(3) ($)
|
Number
of shares or units of shares that have not vested
(#)
|
Market
or payout value of share-based awards that have not vested
($)
|
Market or payout value
of vested share-based awards not paid out or distributed
($) |
|
Vivian Bercovici(4)
|
5,250
3,000 |
40.00
58.70 |
June 9, 2025
May 19, 2026 |
Nil |
Nil |
Nil |
Nil |
|
Haleli Barath(5)
|
9,000 |
100.00 |
February 28, 2026 |
Nil |
Nil |
Nil |
Nil |
|
Brian Schinderle |
9,000 |
100.00 |
February 28, 2026 |
Nil |
Nil |
Nil |
Nil |
|
Moti Marcus(6)
|
9,000 |
6.00 |
September 19, 2027 |
Nil |
Nil |
Nil |
Nil |
|
Einat Zakariya(7)
|
9,000 |
6.00 |
September 19, 2027 |
Nil |
Nil |
Nil |
Nil |
| (1) |
Each Option entitles the holder to purchase one Common Share. |
| (2) |
On February 12, 2021, the Company completed a consolidation of its Common Shares on a 4:1 basis. The figures reported in this table
are presented on a 4:1 post-consolidation basis. |
| (3) |
Calculated using the closing market price of the Common Shares on the CSE on December 31, 2022 of $1.3 and subtracting the exercise
price of in-the-money Options, including unvested. These Options have not been, and may never be, exercised and actual gains, if any,
on exercise will depend on the value of the Common Shares on the date of exercise. |
| (4) |
Ms. Bercovici resigned on September 13, 2022 but remained as a director of IMC Holdings, therefore her options continued to vest
according to the Option Plan. Ms. Bercovici resigned from IMC Holdings on January 8, 2023. |
| (5) |
Ms. Barath resigned on September 13, 2022 but remained as a director of IMC Holdings, therefore her options continued to vest according
to the Option Plan. |
| (6) |
Mr. Marcus appointed on September 13, 2022. |
| (7) |
Ms. Zakariya appointed on September 13, 2022. |
|
Name
|
Option-based
awards – Value vested during the year
($)
|
Share-based
awards – Value vested during the year ($) |
Non-equity
incentive plan compensation – Value earned during the year
($)
|
|
Vivian Bercovici(1)
|
130,200 |
Nil |
Nil |
|
Haleli Barath(2)
|
362,043 |
Nil |
Nil |
|
Brian Schinderle |
362,043 |
Nil |
Nil |
|
Moti Marcus(3)
|
Nil |
Nil |
Nil |
|
Einat Zakariya(4)
|
Nil |
Nil |
Nil |
| (1) |
Ms. Bercovici resigned on September 13, 2022 but remained as a director of IMC Holdings, therefore her options continued to vest
according to the Option Plan. Ms. Bercovici resigned from IMC Holdings on January 8, 2023. |
| (2) |
Ms. Barath resigned on September 13, 2022 but remained as a director of IMC Holdings, therefore her options continued to vest according
to the Option Plan. |
| (3) |
Mr. Marcus appointed on September 13, 2022. |
| (4) |
Ms. Zakariya appointed on September 13, 2022. |
|
Plan Category
|
Number of Securities
to be Issued upon Exercise of Options, Warrants and Rights
(a) |
Weighted – Average
Exercise Price of Outstanding Options, Warrants and Rights
(b) |
Number of Securities
Remaining Available for Future Issuance Under Equity Compensation Plans (excluding securities reflected in column (a))
(c) |
|
Equity Compensation
Plans Approved by Securityholders |
800,535 |
$55.23 |
241,908 |
|
Equity Compensation Plans Not Approved by Securityholders |
Nil |
N/A |
Nil |
|
Total |
800,535 |
$55.23 |
241,908 |
| (1) |
On November 17, 2022, the Company completed a consolidation of its Common Shares on a 10:1 basis. The figures reported in this table
are presented on a 10:1 post-consolidation basis. |
|
Year |
Full Time |
Part Time |
Total | |||
|
Fiscal 2020 |
80 |
- |
80 | |||
|
Fiscal 2021 |
283 |
- |
363 | |||
|
Fiscal 2022 |
153 |
- |
153 |
|
Year |
Israel |
Germany |
Canada |
Total | ||||
|
Fiscal 2020 |
66 |
14 |
- |
80 | ||||
|
Fiscal 2021 |
112 |
15 |
236 |
363 | ||||
|
Fiscal 2022 |
126 |
27 |
- |
153 |
|
Name of Beneficial Owner |
Common Shares Held |
Exercisable Options |
RSUs |
Number of Common Shares Beneficially Owned |
Percent of Outstanding Common Shares |
|||||||||||||||
|
Oren Shuster |
1,872,717 |
105,715 |
- |
153 |
14.58 |
% | ||||||||||||||
|
Marc Lustig |
338,144 |
67,500 |
50,414 |
- |
2.63 |
% | ||||||||||||||
|
Moti Marcus |
- |
- |
- |
- |
- |
|||||||||||||||
|
Einat Zakariya |
61,200 |
- |
- |
- |
0.48 |
% | ||||||||||||||
|
Brian Schinderle |
- |
6,747 |
- |
- |
- |
|||||||||||||||
|
Shai Shemesh |
21,190 |
23,129 |
- |
- |
0.16 |
% | ||||||||||||||
|
Richard Balla |
2,963 |
3,750 |
- |
- |
0.02 |
% | ||||||||||||||
|
Rinat Efrima |
15,000 |
1,665 |
- |
- |
0.12 |
% | ||||||||||||||
|
Total |
2,311,214 |
208,506 |
50,414 |
153 |
17.99 |
% | ||||||||||||||
|
Name |
Aggregate Number of Common Shares |
Percentage of Outstanding Common Shares |
|
Oren Shuster |
1,872,870 |
14.58% |
|
Rafael Gabay |
1,173,869 |
9.14% |
|
Luminera Derm Ltd. |
757,172 |
5.89% |
|
Notes: |
| (1) |
1,872,717 Common Shares are held by Oren Shuster directly and 153 Common Shares are
held indirectly by Ewave Group Ltd., a privately-held entity jointly owned by Mr. Shuster and Mr. Gabay of which Mr. Shuster owns and
controls 50% of the outstanding voting shares. |
| (2) |
1,173,716 Common Shares are held by Rafael Gabay directly and 153 Common Shares are
held indirectly by Ewave Group Ltd., a privately-held entity jointly owned by Mr. Gabay and Mr. Shuster of which Mr. Gabay owns and controls
50% of the outstanding voting shares. |
| • |
Under the Focus Agreement, IMC Holdings retains an option with Messrs. Shuster and Gabay to re-acquire the sold interest in
Focus Medical at its sole discretion and in accordance with Israeli cannabis regulations. |
| • |
The Company is a party to Indemnification Agreement with certain directors and officers of the Company and Trichome to cover certain
tax liabilities, interest and penalties arising from the Trichome Transaction. |
| • |
On August 5, 2022, the Company sold the wholly owned subsidiary of TJAC, Sublime, to a group of purchasers that included current
and former members of the Sublime management team for aggregate proceeds of $100,000 less working capital adjustments, for a final net
purchase price of $89,000. The transaction constituted a “related party transaction” within the meaning of Multilateral Instrument
61-101 - Protection of Minority Security Holders in Special Transactions (“MI
61-101”), however pursuant to Sections 5.5(a) and 5.7(1)(a) of MI 61-101, the transaction is exempt from the formal valuation
and minority shareholder approval requirements of such instrument. |
| • |
The Stalking Horse Purchase Agreement constituted a related party transaction as L5 is an entity controlled by Marc Lustig, who is
a director of Trichome and the Executive Chairman of the Board of the Company. On March 8, 2023, the Company announced that the SISP approved
by the Ontario Superior Court of Justice (Commercial List) did not result in any bids for the going-concern business of Trichome Group.
In addition, L5, controlled by Marc Lustig, advised that it would not complete the proposed transaction contemplated by the Stalking Horse
Share Purchase Agreement. |
| • |
On August 24, 2022, the Company announced that it closed the first tranche of the 2022 Private Placement and on October 5, 2022 announced
that it closed the second tranche of the 2022 Private Placement. Insiders of the Company, led by the Company’s CEO and Director,
and the Company’s CFO, subscribed for 1,563,496 Common Shares for aggregate proceeds of US$782 in the first tranche of the 2022
Private Placement, and the Executive Chairman and Director of the Company, subscribed for 1,112,504 Common Shares for aggregate proceeds
of US$556 in the second tranche of the 2022 Private Placement. As a result of the participation by the CEO, the CFO and the Executive
Chairman and Director of the Company, the 2022 Private Placement was considered a “related party transaction” pursuant to
MI 61-101. The Company relied on Sections 5.5(a) and 5.7(1)(a) of MI 61-101 for exemptions from the requirements to obtain a formal valuation
and minority shareholder approval, respectively, because the fair market value of the Insiders’ participation in the 2022 Private
Placement was below 25% of the Company’s market capitalization for purposes of MI 61-101. |
| • |
On January 16, 2023, the Company announced the closing of the first tranche of the Concurrent Offering comprised of an aggregate
of 1,159,999 Units for aggregate gross proceeds of US$1,500. The Units under the first tranche of the Concurrent Offering were issued
and sold to insiders of the Company, including the Company’s CEO and director of the Company. |
| • |
On January 20, 2023, the Company closed the second tranche of the LIFE Offering comprised of 102,152 Units for an aggregate subscription
price of approximately US$128. The second tranche of the LIFE Offering was comprised of a single subscription by Marc Lustig, a non-independent
director of the Company whose subscription price was satisfied by the settlement of approximately US$128 in debt owed by the Company to
Marc Lustig for certain consulting services previously rendered to the Company. |
| • |
On February 16, 2023, the Company closed the fifth and final tranche of the LIFE Offering. Marc Lustig, a non-independent director
of the Company subscribed for 29,548 Units in the fifth tranche at an aggregate subscription price of US$36,935. Marc Lustig’s subscription
price was satisfied by the settlement of US$37 in debt owed by the Company to the director for certain consulting services previously
rendered by the director to the Company. |
| 2. |
that the contractual partner of the Company is not the defendant, Stroakmont & Atton is not the real purchaser rather a company
named Uniclaro GmbH. |
| 3. |
that the Company allegedly placed an order with Uniclaro GmbH for a total of 4.3 million Clongene COVID-19 tests, of which Uniclaro
GmbH claims to have a payment claim against the Company for a partial delivery of 380,400 Clongene COVID-19 tests in the total amount
of EUR 941,897.20. Uniclaro GmbH has assigned this alleged claim against the Company to Stroakmont & Atton Trading GmbH, and
Stroakmont & Atton Trading GmbH has precautionary declared a set-off against the Company’s claim. |
| 1. |
under the age of 18 years; |
| 2. |
found by a court, in Canada or elsewhere, to be incapable of managing the individual’s own affairs, unless a court, in Canadaor
elsewhere, subsequently finds otherwise; ; |
| 3. |
an undischarged bankrupt; or |
| 4. |
convicted in or out of the Province of British Columbia of an offence in connection with the promotion, formation or management of
a corporation or unincorporated business, or of an offence involving fraud, unless: |
| a. |
the court orders otherwise; |
| b. |
5 years have elapsed since the last to occur of: |
| i. |
the expiration of the period set for suspension of the passing of sentence without a sentence having been passed; |
| ii. |
the imposition of a fine; |
| iii. |
the conclusion of the term of any imprisonment; and |
| iv. |
the conclusion of the term of any probation imposed; or |
| c. |
a pardon was granted or issued, or a record suspension ordered, under the Criminal Records Act (Canada) and the pardon or record
suspension, as the case may be, has not been revoked or ceased to have effect. |
| a. |
Option agreement between IMC Holdings and Focus, dated April 2, 2019, whereby IMC Holdings has an option to purchase, at its sole
discretion, all of the issued and outstanding ordinary shares of Focus at a price equal to NIS 765.67 per ordinary share for total consideration
of NIS 2,756,500 until April 2029. |
| b. |
Services Agreement dated April 2, 2019 and as amended on January 1, 2021, between IMC Holdings and Focus, as further described in
“Business Overview – Economic Dependence”. |
| c. |
Agency agreement dated May 5, 2021 between the Company Roth Canada, ULC, pursuant to which Roth Canada, ULC acted as the sole agent
in Canada in respect to an offering of Common Shares and Warrant completed in May 2021 for aggregate gross proceeds of US$35,000,000.
|
| d. |
The warrant indenture between the Company and Odyssey Trust Company, dated January 30, 2023, entered into in respect of Warrants
issued under the LIFE Offering. |
| e. |
The warrant indenture between the Company and Odyssey Trust Company, dated February 7, 2023, entered into in respect of Warrants
issued under the LIFE Offering. |
| f. |
The warrant indenture between the Company and Odyssey Trust Company, dated February 16, 2023, entered into in respect of Warrants
issued under the LIFE Offering. |
|
(i) |
the entity carries on a prescribed business
activity, |
|
(ii) |
the non-Canadian could, as a result of the
investment, have access to, or direct the use of, material non-public technical information or material assets, and |
|
(iii) |
the non-Canadian would have, as a result
of the investment, |
|
(A) |
the power to appoint or nominate any person
who has the capacity to direct the business and affairs of the entity, such as a member of the board of directors or of senior management,
or |
|
(B) |
prescribed special rights with respect to
the entity. |
| A. |
a citizen or individual resident of the United States; |
| B. |
a corporation (or other entity classified as a corporation for U.S. federal income tax purposes) organized under the laws of the
United States, any state thereof or the District of Columbia; |
| C. |
an estate whose income is subject to U.S. federal income taxation regardless of its source; or |
| D. |
a trust that (1) is subject to the primary supervision of a court within the United States and the control of one or more U.S. persons
for all substantial decisions or (2) has a valid election in effect under applicable Treasury Regulations to be treated as a U.S. person.
|
| (i) |
are resident solely in the United States for the purposes of the Canada-U.S. Tax Convention; |
| (ii) |
are entitled to the benefits of the Canada-U.S. Tax Convention and is a “qualifying person” within the meaning of the
Canada-U.S. Tax Convention; |
| (iii) |
hold all Common Shares as capital property and as beneficial owner; |
| (iv) |
hold no Common Shares that are “taxable Canadian property” (as defined in the Tax Act) of the holder; |
| (v) |
deal at arm’s length with and are not affiliated with the Company; |
| (vi) |
do not use or hold and are not deemed to use or hold any Common Shares in the course of a business or part of a business carried
on in Canada; |
| (vii) |
did not, do not and will not have a permanent establishment in Canada within the meaning of the Canada-U.S. Tax Convention;
|
| (viii) |
did not acquire Common Shares by virtue of employment; |
| (ix) |
are not financial institutions, authorized foreign banks, partnerships or trusts for the purposes of the Tax Act; and |
| (x) |
are not insurers that carry on business in Canada and elsewhere; |
| (i) |
the holder, persons with whom the holder does not deal at arm’s length, or any partnership in which the holder or persons with
whom the holder did not deal at arm’s length holds a membership interest directly or indirectly through one or more partnerships,
alone or in any combination, owned 25% or more of the issued shares of any class of the capital stock of the Company; and |
| (ii) |
more than 50% of the fair market value of the Common Shares was derived directly or indirectly from, or from any combination of,
real or immovable property situated in Canada, “Canadian resource properties” (as defined in the Tax Act), “timber resource
properties” (as defined in the Tax Act), or options in respect of or interests in, or for civil law a right, in such properties.
|
|
December 31, 2022(1)
|
December 31, 2021(1)
| |
|
Audit fees(2)
|
$315 |
$894 |
|
Audit-related fees(3)
|
$45 |
- |
|
Tax fees(4)
|
$60 |
$17 |
|
All other fees(5)
|
- |
- |
|
Total fees |
$420 |
$911 |
| (1) |
Amounts are stated in thousands USD and are from continuing operations. |
| (2) |
Audit fees consist of the aggregate fees billed for the audit or review of the Company’s annual and quarterly financial statements
that are normally provided in connection with statutory and regulatory filings or engagements. |
| (3) |
Audit-related fees consist of the aggregate fees billed for assurance and related services that are reasonably related to the performance
of the audit or review of the Company’s financial statements and are not reported as audit fees. |
| (4) |
For tax compliance, tax advice and tax planning. |
| (5) |
For products and services other than the audit fees, audit-related fees and tax fees described above. |
| (6) |
Amounts stated in the table above are not including 2022 audit fees related to the deconsolidated Trichome Group in the amount of
$331,000. |
|
Consolidated Financial Statements for the Years Ended December
31, 2022 and 2021 |
|
Independent Auditors’ Reports |
|
Consolidated Statements of Financial Position |
|
Consolidated Statements of Net Loss and Comprehensive Loss |
|
Consolidated Statements of Changes in Shareholders’ Equity |
|
Consolidated Statements of Cash Flows |
|
Notes to the Consolidated Financial Statements |
|
Description |
Page | |
|
Consolidated Financial Statements and Notes |
F-1 - F-81 |
|
Exhibit
|
|
|
No. Item |
Description of Exhibit
|
|
| |
| 4.4 |
|
|
101.INS |
XBRL Instant Document
|
|
101.SCH |
XBRL Taxonomy Extension Schema Document
|
|
101.CAL |
XBLR Taxonomy Extension Calculation Linkbase Document
|
|
101.DEF |
XBRL Taxonomy Extension Definition Linkbase
|
|
101.LAB |
XBRL Taxonomy Extension Label Linkbase
|
|
101.PRE |
XBRL Taxonomy Extension Presentation Linkbase
|
|
104 |
Cover Page Interactive Data File – (formatted as Inline XBRL and contained in
Exhibit 101) |
|
IM Cannabis Corp.
|
|||
|
Date: March 29, 2023
|
By: |
/s/ Shai Shemesh | |
| Name: Shai Shemesh | |||
| Title: Chief Financial Officer | |||

|
Page
|
|
|
Report of Independent Registered Public Accounting Firm (PCAOB ID:
|
F - 2
|
|
F - 3 – F - 4
|
|
|
F - 5 – F - 6
|
|
|
F - 7 – F - 8
|
|
|
F - 9 – F - 11
|
|
|
F - 12 – F - 81
|
 |
Kost Forer Gabbay & Kasierer
144 Menachem Begin Road, Building A,
Tel-Aviv 6492102, Israel
|
Tel: +972-3-6232525
Fax: +972-3-5622555
ey.com
|
|
|
|
A Member of Ernst & Young Global
|
|
We have served as the Company's auditor since 2018.
|
|
|
|
March 29, 2023
|
|
December 31,
|
|||||||||||
|
Note
|
2022
|
2021
|
|||||||||
|
ASSETS
|
|||||||||||
|
CURRENT ASSETS:
|
|||||||||||
|
Cash and cash equivalents
|
$
|
|
$
|
|
|||||||
|
Trade receivables
|
6
|
|
|
||||||||
|
Advances to suppliers
|
|
|
|||||||||
|
Other accounts receivable
|
7
|
|
|
||||||||
|
Loans receivable
|
15e
|
|
|
|
|||||||
|
Biological assets
|
8
|
|
|
||||||||
|
Inventory
|
9
|
|
|
||||||||
|
|
|
||||||||||
|
NON-CURRENT ASSETS:
|
|||||||||||
|
Property, plant and equipment, net
|
10
|
|
|
||||||||
|
Investments in affiliates
|
15c
|
|
|
|
|||||||
|
Advance payment for intangible assets of pharmacy
|
5
|
|
|
||||||||
|
Derivative assets
|
|
|
|||||||||
|
Right-of-use assets, net
|
12
|
|
|
||||||||
|
Deferred tax assets, net
|
17
|
|
|
||||||||
|
Intangible assets, net
|
5, 11
|
|
|
||||||||
|
Goodwill
|
5, 11
|
|
|
||||||||
|
|
|
||||||||||
|
Total assets
|
$
|
|
$
|
|
|||||||
|
December 31,
|
|||||||||||
|
Note
|
2022
|
2021
|
|||||||||
|
LIABILITIES AND EQUITY
|
|||||||||||
|
CURRENT LIABILITIES:
|
|||||||||||
|
Trade payables
|
$
|
|
$
|
|
|||||||
|
Bank loans and others
|
1
|
|
|
||||||||
|
Other accounts payable and accrued expenses
|
14
|
|
|
||||||||
|
Accrued purchase consideration liabilities
|
5
|
|
|
||||||||
|
Current maturities of operating lease liabilities
|
12
|
|
|
||||||||
|
|
|
||||||||||
|
NON-CURRENT LIABILITIES:
|
|||||||||||
|
Warrants measured at fair value
|
15
|
|
|
||||||||
|
Operating lease liabilities
|
12
|
|
|
||||||||
|
Long-term loans
|
|
|
|||||||||
|
Employee benefit liabilities, net
|
13
|
|
|
||||||||
|
Deferred tax liability, net
|
17
|
|
|
||||||||
|
|
|
||||||||||
|
Total liabilities
|
|
|
|||||||||
|
EQUITY ATTRIBUTABLE TO EQUITY HOLDERS OF THE COMPANY:
|
18
|
||||||||||
|
Share capital and premium
|
|
|
|||||||||
|
Treasury Stock
|
|
|
(
|
)
|
|||||||
|
Translation reserve
|
|
|
|||||||||
|
Reserve from share-based payment transactions
|
|
|
|||||||||
|
Accumulated deficit
|
(
|
)
|
(
|
)
|
|||||||
|
Total equity attributable to shareholders of the Company
|
|
|
|||||||||
|
Non-controlling interests
|
|
|
|||||||||
|
Total equity
|
|
|
|||||||||
|
Total equity and liabilities
|
$
|
|
$
|
|
|||||||
|
March 29, 2023
|
/s/ Marc Lustig | /s/ Oren Shuster | /s/ Shai Shemesh | |||
|
Date of approval of the
|
Marc Lustig
|
Oren Shuster
|
Shai Shemesh
|
|||
|
financial statements
|
Chairman of the Board
|
Chief Executive Officer
|
Chief Financial Officer
|
AND OTHER COMPREHENSIVE INCOME
|
Year ended
December 31,
|
|||||||||||||||
|
Note
|
2022
|
2021 (*)
|
|
2020
|
|||||||||||
|
Revenues
|
$
|
|
$
|
|
$
|
|
|||||||||
|
Cost of revenues
|
|
|
|
||||||||||||
|
Gross profit before fair value adjustments
|
|
|
|
||||||||||||
|
Fair value adjustments:
|
|||||||||||||||
|
Unrealized change in fair value of biological assets
|
(
|
)
|
|
|
|||||||||||
|
Realized fair value adjustments on inventory sold in the year
|
(
|
)
|
(
|
)
|
(
|
)
|
|||||||||
|
Total fair value adjustments
|
(
|
)
|
(
|
)
|
|
||||||||||
|
Gross profit after fair value adjustments
|
|
|
|
||||||||||||
|
General and administrative expenses
|
|
|
|
||||||||||||
|
Selling and marketing expenses
|
|
|
|
||||||||||||
|
Restructuring expenses
|
1
|
|
|
|
|||||||||||
|
Share-based compensation
|
18
|
|
|
|
|||||||||||
|
Total operating expenses
|
|
|
|
||||||||||||
|
Operating loss
|
(
|
)
|
(
|
)
|
(
|
)
|
|||||||||
|
Finance income
|
15
|
|
|
|
|||||||||||
|
Finance expenses
|
(
|
)
|
(
|
)
|
(
|
)
|
|||||||||
|
Finance income (expense), net
|
|
|
(
|
)
|
|||||||||||
|
Loss before income taxes
|
(
|
)
|
(
|
)
|
(
|
)
|
|||||||||
|
Income tax expense (benefit)
|
17
|
(
|
)
|
|
|
||||||||||
|
Net loss from continuing operations
|
(
|
)
|
(
|
)
|
(
|
)
|
|||||||||
|
Net loss from discontinued operations, net of tax
|
24 |
(
|
)
|
(
|
)
|
|
|||||||||
|
Net loss
|
$
|
(
|
)
|
$
|
(
|
)
|
$
|
(
|
)
|
||||||
|
Year ended
December 31,
|
|||||||||||||||
|
Note
|
2022
|
2021 (*)
|
|
2020
|
|||||||||||
|
Other comprehensive income that will not be reclassified to profit or loss in subsequent periods:
|
|||||||||||||||
|
Remeasurement gain (loss) on defined benefit plans
|
|
|
(
|
)
|
|||||||||||
|
Exchange differences on translation to presentation currency
|
(
|
)
|
|
|
|||||||||||
|
Total other comprehensive income that will not be reclassified to profit or loss in subsequent periods
|
(
|
)
|
|
|
|||||||||||
|
Other comprehensive income that will be reclassified to profit or loss in subsequent periods:
|
|||||||||||||||
|
Adjustments arising from translating financial statements of foreign operation
|
(
|
)
|
|
(
|
)
|
||||||||||
|
Total other comprehensive income (loss)
|
(
|
)
|
|
|
|||||||||||
|
Total comprehensive loss
|
(
|
)
|
(
|
)
|
(
|
)
|
|||||||||
|
Net loss attributable to:
|
|||||||||||||||
|
Equity holders of the Company
|
(
|
)
|
(
|
)
|
(
|
)
|
|||||||||
|
Non-controlling interests
|
(
|
)
|
(
|
)
|
(
|
)
|
|||||||||
|
(
|
)
|
(
|
)
|
(
|
)
|
||||||||||
|
Total comprehensive income (loss) attributable to:
|
|||||||||||||||
|
Equity holders of the Company
|
(
|
)
|
(
|
)
|
(
|
)
|
|||||||||
|
Non-controlling interests
|
(
|
)
|
(
|
)
|
|
||||||||||
|
$
|
(
|
)
|
$
|
(
|
)
|
$
|
(
|
)
|
|||||||
|
Earnings (loss) per share attributable to equity holders of the Company from continuing operations:
|
20
|
||||||||||||||
|
Basic earnings (loss) per share (in CAD)
|
$
|
(
|
)
|
$
|
|
$
|
(
|
)
|
|||||||
|
Diluted loss per share (in CAD)
|
$
|
(
|
)
|
$
|
(
|
)
|
$
|
(
|
)
|
||||||
|
Loss per share attributable to equity holders of the Company from discontinued operations:
|
|||||||||||||||
|
Basic and diluted loss per share (in CAD)
|
$
|
(
|
)
|
$
|
(
|
)
|
$
|
|
|||||||
|
Loss per share attributable to equity holders of the Company from net loss:
|
|||||||||||||||
|
Basic earnings (loss) per share (in CAD)
|
$
|
(
|
)
|
$
|
(
|
)
|
$
|
(
|
)
|
||||||
|
Diluted loss per share (in CAD)
|
$
|
(
|
)
|
$
|
(
|
)
|
$
|
(
|
)
|
||||||
CONSOLIDATED STATEMENTS OF CHANGES IN EQUITY
|
Share capital and premium*)
|
Treasury Stock
|
Reserve from share-based payment transactions
|
Translation reserve
|
Accumulated deficit
|
Total
|
Non-controlling interests
|
Total
equity
|
|||||||||||||||||||||||||
|
Balance as of January 1, 2020
|
$
|
|
$
|
|
$
|
|
$
|
|
$
|
(
|
)
|
$
|
|
$
|
|
$
|
|
|||||||||||||||
|
Net loss
|
|
|
|
|
(
|
)
|
(
|
)
|
(
|
)
|
(
|
)
|
||||||||||||||||||||
|
Total other comprehensive income (loss)
|
|
|
|
|
(
|
)
|
|
|
|
|||||||||||||||||||||||
|
Total comprehensive income (loss)
|
|
|
|
|
(
|
)
|
(
|
)
|
|
(
|
)
|
|||||||||||||||||||||
|
Exercise of warrants and compensation options
|
|
|
|
|
|
|
|
|
||||||||||||||||||||||||
|
Exercise of options
|
|
|
(
|
)
|
|
|
|
|
|
|||||||||||||||||||||||
|
Share-based compensation
|
|
|
|
|
|
|
|
|
||||||||||||||||||||||||
|
Expired options
|
|
|
(
|
)
|
|
|
|
|
|
|||||||||||||||||||||||
|
Balance as of January 1, 2021
|
$
|
|
$
|
|
$
|
|
$
|
|
$
|
(
|
)
|
$
|
|
$
|
|
$
|
|
|||||||||||||||
|
Net loss
|
|
|
|
|
(
|
)
|
(
|
)
|
(
|
)
|
(
|
)
|
||||||||||||||||||||
|
Total other comprehensive income
|
|
|
|
|
|
|
|
|
||||||||||||||||||||||||
|
Total comprehensive income (loss)
|
|
|
|
|
(
|
)
|
(
|
)
|
(
|
)
|
(
|
)
|
||||||||||||||||||||
|
Issuance of common shares, net of issuance costs of $
|
|
|
|
|
|
|
|
|
||||||||||||||||||||||||
|
Purchase of treasury common shares
|
|
(
|
)
|
|
|
|
(
|
)
|
|
(
|
)
|
|||||||||||||||||||||
|
Exercise of warrants and compensation options
|
|
|
|
|
|
|
|
|
||||||||||||||||||||||||
|
Exercise of options
|
|
|
(
|
)
|
|
|
|
|
|
|||||||||||||||||||||||
|
Share-based compensation
|
|
|
|
|
|
|
|
|
||||||||||||||||||||||||
|
Expired options
|
|
|
(
|
)
|
|
|
|
|
|
|||||||||||||||||||||||
|
Balance as of December 31, 2021
|
$
|
|
$
|
(
|
)
|
$
|
|
$
|
|
$
|
(
|
)
|
$
|
|
$
|
|
$
|
|
||||||||||||||
|
Share capital and premium*)
|
Treasury Stock
|
Reserve from share-based payment transactions
|
Translation reserve
|
Accumulated deficit
|
Total
|
Non-controlling interests
|
Total
equity
|
|||||||||||||||||||||||||
|
Balance as of January 1, 2022
|
$
|
|
$
|
(
|
)
|
$
|
|
$
|
|
$
|
(
|
)
|
$
|
|
$
|
|
$
|
|
||||||||||||||
|
Net loss
|
|
|
|
|
(
|
)
|
(
|
)
|
(
|
)
|
(
|
)
|
||||||||||||||||||||
|
Total other comprehensive income (loss)
|
|
|
|
(
|
)
|
|
(
|
)
|
(
|
)
|
(
|
)
|
||||||||||||||||||||
|
Total comprehensive loss
|
|
|
|
(
|
)
|
(
|
)
|
(
|
)
|
(
|
)
|
(
|
)
|
|||||||||||||||||||
|
Issuance of treasury common shares
|
|
|
|
|
|
|
|
|
||||||||||||||||||||||||
|
Issuance of shares, net of issuance costs of $
|
|
|
|
|
|
|
|
|
||||||||||||||||||||||||
|
Exercise of options
|
|
|
(
|
)
|
|
|
|
|
|
|||||||||||||||||||||||
|
Share-based compensation
|
|
|
|
|
|
|
|
|
||||||||||||||||||||||||
|
Expired options
|
|
|
(
|
)
|
|
|
|
|
|
|||||||||||||||||||||||
|
Balance as of December 31, 2022
|
$
|
|
$
|
|
$
|
|
$
|
|
$
|
(
|
)
|
$
|
|
$
|
|
$
|
|
|||||||||||||||
CONSOLIDATED STATEMENTS OF CASH FLOWS
|
Year ended
December 31,
|
||||||||||||
|
2022
|
2021
|
2020
|
||||||||||
|
Cash provided from operating activities:
|
||||||||||||
|
Net loss
|
$
|
(
|
)
|
$
|
(
|
)
|
$
|
(
|
)
|
|||
|
Adjustments for non-cash items:
|
||||||||||||
|
Unrealized gain on changes in fair value of biological assets
|
(
|
)
|
(
|
)
|
(
|
)
|
||||||
|
Fair value adjustment on sale of inventory
|
|
|
|
|||||||||
|
Fair value adjustment on Warrants, Investments, and Accounts Receivable
|
(
|
)
|
(
|
)
|
|
|||||||
|
Depreciation of property, plant and equipment
|
|
|
|
|||||||||
|
Amortization of intangible assets
|
|
|
|
|||||||||
|
Depreciation of right-of-use assets
|
|
|
|
|||||||||
|
Impairment of goodwill
|
|
|
|
|||||||||
|
Impairment of property, plant and equipment
|
|
|
|
|||||||||
|
Impairment of intangible assets
|
|
|
|
|||||||||
|
Impairment of right-of-use assets
|
|
|
|
|||||||||
|
Finance income, net
|
|
|
|
|||||||||
|
Deferred tax payments (benefit), net
|
(
|
)
|
|
(
|
)
|
|||||||
|
Share-based payments
|
|
|
|
|||||||||
|
Share based acquisition costs related to business combination
|
|
|
|
|||||||||
|
Revaluation of other accounts receivable
|
|
|
|
|||||||||
|
Restructuring expenses
|
|
|
|
|||||||||
|
|
(
|
)
|
|
|||||||||
|
Changes in non-cash working capital:
|
||||||||||||
|
Increase (decrease) in trade receivables, net
|
|
(
|
)
|
(
|
)
|
|||||||
|
Increase (decrease) in other accounts receivable and advances to suppliers
|
|
|
(
|
)
|
||||||||
|
Decrease in biological assets, net of fair value adjustments
|
|
|
|
|||||||||
|
Increase (decrease) in inventory, net of fair value adjustments
|
|
(
|
)
|
(
|
)
|
|||||||
|
Increase in trade payables
|
|
|
|
|||||||||
|
Changes in employee benefit liabilities, net
|
(
|
)
|
|
|
||||||||
|
Increase in other accounts payable and accrued expenses
|
|
|
|
|||||||||
|
|
(
|
)
|
(
|
)
|
||||||||
|
Taxes paid
|
(
|
)
|
(
|
)
|
(
|
)
|
||||||
|
Net cash used in operating activities
|
(
|
)
|
(
|
)
|
(
|
)
|
||||||
CONSOLIDATED STATEMENTS OF CASH FLOWS
|
Year ended
December 31,
|
||||||||||||
|
2022
|
2021
|
2020
|
||||||||||
|
Cash flows from investing activities:
|
||||||||||||
|
Purchase of property, plant and equipment
|
$
|
(
|
)
|
$
|
(
|
)
|
$
|
(
|
)
|
|||
|
Proceeds from sales of property, plant and equipment
|
|
|
|
|||||||||
|
Proceeds from loans receivable
|
|
|
|
|||||||||
|
Purchase of intangible assets
|
|
(
|
)
|
(
|
)
|
|||||||
|
Acquisition of businesses, net of cash acquired
|
|
(
|
)
|
|
||||||||
|
Deconsolidation of subsidiary (see Note 24)
|
(
|
)
|
|
|
||||||||
|
Investments in financial assets
|
|
(
|
)
|
(
|
)
|
|||||||
|
Proceeds from sale of investment
|
|
|
|
|||||||||
|
Proceeds from (investment in) restricted deposits
|
|
|
(
|
)
|
||||||||
|
Net cash used in investing activities
|
(
|
)
|
(
|
)
|
(
|
)
|
||||||
|
Cash provided by financing activities:
|
||||||||||||
|
Proceeds from issuance of share capital, net of issuance costs
|
|
|
|
|||||||||
|
Proceeds from issuance of warrants measured at fair value
|
|
|
|
|||||||||
|
Proceeds from exercise of warrants
|
|
|
|
|||||||||
|
Proceeds from exercise of options
|
|
|
|
|||||||||
|
Repayment of lease liability
|
(
|
)
|
(
|
)
|
(
|
)
|
||||||
|
Payment of lease liability interest
|
(
|
)
|
(
|
)
|
(
|
)
|
||||||
|
Proceeds from loans
|
|
|
|
|||||||||
|
Repayment of loans
|
(
|
)
|
|
|
||||||||
|
Interest paid
|
(
|
)
|
(
|
)
|
|
|||||||
|
Net cash provided by financing activities
|
|
|
|
|||||||||
CONSOLIDATED STATEMENTS OF CASH FLOWS
|
Year ended
December 31,
|
||||||||||||
|
2022
|
2021
|
2020
|
||||||||||
|
Effect of foreign exchange on cash and cash equivalents
|
$
|
(
|
)
|
$
|
(
|
)
|
$
|
|
||||
|
Increase (decrease) in cash and cash equivalents
|
(
|
)
|
|
(
|
)
|
|||||||
|
Cash and cash equivalents at beginning of year
|
|
|
|
|||||||||
|
Cash and cash equivalents at end of year
|
$
|
|
$
|
|
$
|
|
||||||
|
Supplemental disclosure of non-cash activities:
|
||||||||||||
|
Right-of-use asset recognized with corresponding lease liability
|
$
|
|
$
|
|
$
|
|
||||||
|
Conversion of warrant and compensation options into common shares
|
$
|
|
$
|
|
$
|
|
||||||
|
Issuance of shares in payment of purchase consideration liability
|
$
|
|
$
|
|
$
|
|
||||||
IM CANNABIS CORP. AND ITS SUBSIDIARIES
| NOTE 1:- |
GENERAL
|
| a. |
Corporate information:
|
|
NOTE 1:-
|
GENERAL (Cont.)
|
|
NOTE 1:-
|
GENERAL (Cont.)
|
|
NOTE 1:-
|
GENERAL (Cont.)
|
|
NOTE 1:-
|
GENERAL (Cont.)
|
| b. |
Approval of consolidated financial statements:
|
| c. |
Definitions:
|
|
The Company, or IMCC
|
-
|
IM Cannabis Corp.
|
|
The Group
|
-
|
IM Cannabis Corp., its Subsidiaries
|
|
Subsidiaries
|
-
|
Companies that are controlled by the Company (as defined in IFRS 10) and whose accounts are consolidated with those of the Company
|
|
CAD or $
|
-
|
Canadian Dollar
|
|
NIS
|
-
|
New Israeli Shekel
|
|
NOTE 2:-
|
SIGNIFICANT ACCOUNTING POLICIES
|
| a. |
Basis of presentation:
|
| - |
Financial instruments which are presented at fair value through profit or loss.
|
| - |
Biological assets which are presented at fair value less cost to sell up to the point of harvest.
|
| b. |
Consolidated financial statements:
|
| NOTE 2:- |
SIGNIFICANT ACCOUNTING POLICIES (Cont.)
|
|
Percentage ownership
|
||||||||
|
Subsidiaries
|
2022
|
2021
|
||||||
|
I.M.C. Holdings Ltd. (”IMC”)
|
|
%
|
|
%
|
||||
|
Focus Medical Herbs Ltd. ("Focus")
|
|
%
|
|
%
|
||||
|
I.M.C. Pharma Ltd.
|
|
%
|
|
|||||
|
I.M.C.C. Medical Herbs Ltd.
|
|
%
|
|
%
|
||||
|
I.M.C Farms Israel Ltd. ("IMC Farms")
|
|
%
|
|
%
|
||||
|
I.M.C Ventures Ltd. ("IMC Ventures")
|
|
%
|
|
%
|
||||
|
I.M.C - International Medical Cannabis Portugal Unipessoal Lda) ***)
|
|
|
%
|
|||||
|
Adjupharm GmbH (“Adjupharm”)
|
|
%
|
|
%
|
||||
|
R.A. Yarok Pharm Ltd. (“Pharm Yarok”)
|
|
%
|
|
%
|
||||
|
Rosen High Way Ltd. (“Rosen High Way”)
|
|
%
|
|
%
|
||||
|
High Way Shinua Ltd. (“HW Shinua”)
|
|
%
|
|
%
|
||||
|
Revoly Trading and Marketing Ltd. (“Vironna”)
|
|
%
|
|
%
|
||||
|
Oranim Plus Pharm LTD.
|
|
%
|
|
%
|
||||
|
Oranim Pharm
|
|
%
|
|
%
|
||||
|
Trichome Financial Corp. (“Trichome”)
|
|
) |
|
%
|
||||
|
Trichome Financial Cannabis GP Inc.
|
|
) |
|
%
|
||||
|
Trichome Financial Cannabis Manager Inc.
|
|
) |
|
%
|
||||
|
Trichome Asset Funding Corp.
|
|
) |
|
%
|
||||
|
Trichome JWC Acquisition Corp. (“TJAC”)
|
|
) |
|
%
|
||||
|
Trichome Retail Corp.
|
|
) |
|
%
|
||||
|
MYM Nutraceuticals Inc. (“MYM”)
|
|
) |
|
%
|
||||
|
SublimeCulture Inc.
|
|
) |
|
%
|
||||
|
CannaCanada Inc.
|
|
) |
|
%
|
||||
|
MYM International Brands Inc.
|
|
) |
|
%
|
||||
|
Highland Grow Inc.
|
|
) |
|
%
|
||||
| *) |
The Company does not hold directly interest or voting rights in Focus. The Company's wholly-owned subsidiary holds an option to buy the ownership of the
|
| **) |
Deconsolidated effective November 7, 2022, when Trichome filed to commence proceedings under the Companies’ Creditors Arrangement Act (CCAA) (see Note 1).
|
| ***) |
Dissolved as of December 31, 2022.
|
| NOTE 2:- |
SIGNIFICANT ACCOUNTING POLICIES (Cont.)
|
| - |
Derecognizes the subsidiary's assets (including goodwill) and liabilities.
|
| - |
Derecognizes the carrying amount of non-controlling interests.
|
| - |
Derecognizes the adjustments arising from translating financial statements carried to equity.
|
| - |
Recognizes the fair value of the consideration received.
|
| - |
Recognizes the fair value of any remaining investment.
|
| - |
Reclassifies the components previously recognized in other comprehensive income (loss) on the same basis as would be required if the subsidiary had directly disposed of the related assets or liabilities.
|
| - |
Recognizes any resulting difference (surplus or deficit) as gain or loss.
|
| c. |
Business combinations and goodwill:
|
|
NOTE 2:-
|
SIGNIFICANT ACCOUNTING POLICIES (Cont.)
|
| d. |
Functional currency, presentation currency and foreign currency:
|
| 1. |
Functional currency and presentation currency:
|
| 2. |
Transactions, assets and liabilities in foreign currency:
|
| NOTE 2:- |
SIGNIFICANT ACCOUNTING POLICIES (Cont.)
|
| e. |
Cash equivalents:
|
| f. |
Fair value measurement:
|
|
Level 1
|
-
|
quoted prices (unadjusted) in active markets for identical assets or liabilities.
|
|
Level 2
|
-
|
inputs other than quoted prices included within Level 1 that are observable directly or indirectly.
|
|
Level 3
|
-
|
inputs that are not based on observable market data (valuation techniques which use inputs that are not based on observable market data).
|
| NOTE 2:- | SIGNIFICANT ACCOUNTING POLICIES (Cont.) |
| g. |
Biological assets:
|
| NOTE 2:- |
SIGNIFICANT ACCOUNTING POLICIES (Cont.)
|
| h. |
Inventory:
|
| i. |
Property, plant and equipment, net:
|
|
%
|
Mainly %
|
|||||
|
Buildings
|
|
|
||||
|
Greenhouse production equipment
|
|
|
||||
|
Greenhouse structure
|
|
|
||||
|
Motor vehicles
|
|
|
||||
|
Computer, software and equipment
|
|
|
||||
|
Leasehold improvements
|
See below
|
See below
|
| NOTE 2:- |
SIGNIFICANT ACCOUNTING POLICIES (Cont.)
|
| j. |
Intangible assets:
|
|
Years
|
||
|
Customer relationship
|
|
|
|
Brand name
|
|
|
|
Other intangibles
|
|
| k. |
Impairment of non-financial assets:
|
| NOTE 2:- |
SIGNIFICANT ACCOUNTING POLICIES (Cont.)
|
| l. |
Revenue recognition:
|
| NOTE 2:- |
SIGNIFICANT ACCOUNTING POLICIES (Cont.)
|
| a) |
The reason for the bill-and-hold arrangement is substantive (for example, the customer has requested the arrangement);
|
| b) |
The product is identified separately as belonging to the customer;
|
| c) |
The product currently is ready for physical delivery to the customer;
|
| d) |
The Group does not have the ability to use the product by selling it or delivering it to another customer.
|
| m. |
Leases:
|
| NOTE 2:- |
SIGNIFICANT ACCOUNTING POLICIES (Cont.)
|
| NOTE 2:- |
SIGNIFICANT ACCOUNTING POLICIES (Cont.)
|
| n. |
Financial instruments:
|
| 1. |
Financial assets:
|
| - |
The Group’s business model for managing financial assets; and
|
| - |
The contractual cash flow terms of the financial asset.
|
| NOTE 2:- |
SIGNIFICANT ACCOUNTING POLICIES (Cont.)
|
| - |
The contractual rights to the cash flows from the financial asset has expired; or
|
| - |
The Group has transferred substantially all the risks and rewards deriving from the contractual rights to receive cash flows from the financial asset or has neither transferred nor retained substantially all the risks and rewards of the asset, but has transferred control of the asset; or
|
| - |
The Group has retained its contractual rights to receive cash flows from the financial asset but has assumed a contractual obligation to pay the cash flows in full without material delay to a third party.
|
| 2. |
Financial liabilities:
|
| 3. |
Issue of a unit of securities:
|
| NOTE 2:- |
SIGNIFICANT ACCOUNTING POLICIES (Cont.)
|
| o. |
Employee benefit liabilities:
|
| 1. |
Short-term employee benefits:
|
| 2. |
Post-employment benefits:
|
| NOTE 2:- |
SIGNIFICANT ACCOUNTING POLICIES (Cont.)
|
| p. |
Share-based payment transactions:
|
| q. |
Provisions:
|
| NOTE 2:- |
SIGNIFICANT ACCOUNTING POLICIES (Cont.)
|
| r. |
Taxes on income:
|
| s. |
Earnings per share:
|
| t. |
Treasury shares:
|
| NOTE 2:- |
SIGNIFICANT ACCOUNTING POLICIES (Cont.)
|
| u. |
Operating segments:
|
| v. |
Discontinued operations:
|
| NOTE 3:- |
SIGNIFICANT ACCOUNTING ESTIMATES AND ASSUMPTIONS USED IN THE PREPARATION OF THE FINANCIAL STATEMENTS
|
| a. |
Judgments:
|
| - |
Determining the fair value of share-based payment transactions:
|
| - |
Discount rate for a lease liability:
|
| NOTE 3:- |
SIGNIFICANT ACCOUNTING ESTIMATES AND ASSUMPTIONS USED IN THE PREPARATION OF THE FINANCIAL STATEMENTS (Cont.)
|
| b. |
Estimates and assumptions:
|
| - |
Legal claims:
|
| - |
Deferred tax assets:
|
| - |
Impairment of goodwill:
|
| NOTE 3:- |
SIGNIFICANT ACCOUNTING ESTIMATES AND ASSUMPTIONS USED IN THE PREPARATION OF THE FINANCIAL STATEMENTS (Cont.)
|
| - |
Determining the fair value of an unquoted financial assets and liabilities:
The fair value of unquoted financial assets in Level 3 of the fair value hierarchy is determined using valuation techniques, generally using future cash flows discounted at current rates applicable for items with similar terms and risk characteristics. changes in estimated future cash flows and estimated discount rates, after consideration of risks such as liquidity risk, credit risk and volatility, are liable to affect the fair value of these assets.
|
| - |
Loss of control of subsidiary
On November 7, 2022, Trichome filed a petition with the Superior Court of Ontario for protection under the Companies’ Creditors Arrangement Act (“CCAA”) in order to restructure its business and financial affairs. Management applied judgement in assessing whether this event represented a loss of control of Trichome. On filing of CCAA, which included a request for an order to approve a sale and investment solicitation process and to approve a stalking horse agreement of purchase and sale, management concluded that the Company ceased to have the power to direct the relevant activity of Trichome because substantive rights were granted to other parties through the CCAA proceedings that restricted the decision making ability of the Company to the extent that the Company was unable to demonstrate power over Trichome. As a result, the Company accounted for a loss in control and Trichome was deconsolidated on November 17, 2022 (see Note 1 and Note 24).
|
| NOTE 4:- |
DISCLOSURE OF NEW STANDARDS IN THE PERIOD PRIOR TO THEIR ADOPTION
|
| a. |
Amendment to IAS 1, "Presentation of Financial Statements":
|
| • |
Only covenants with which an entity must comply on or before the reporting date will affect a liability's classification as current or non-current.
|
| • |
An entity should provide disclosure when a liability arising from a loan agreement is classified as non-current and the entity's right to defer settlement is contingent on compliance with future covenants within twelve months from the reporting date. This disclosure is required to include information about the covenants and the related liabilities. The disclosures must include information about the nature of the future covenants and when compliance is applicable, as well as the carrying amount of the related liabilities. The purpose of this information is to allow users to understand the nature of the future covenants and to assess the risk that a liability classified as non-current could become repayable within twelve months. Furthermore, if facts and circumstances indicate that an entity may have difficulty in complying with such covenants, those facts and circumstances should be disclosed.
|
| b. |
Amendment to IAS 8, "Accounting Policies, Changes to Accounting Estimates and Errors":
|
| NOTE 4:- |
DISCLOSURE OF NEW STANDARDS IN THE PERIOD PRIOR TO THEIR ADOPTION (Cont.)
|
| c. |
Amendment to IAS 12, "Income Taxes":
|
| d. |
Amendment to IAS 1, "Disclosure of Accounting Policies":
|
| NOTE 5:- |
BUSINESS COMBINATIONS
|
|
NOTE 5:-
|
BUSINESS COMBINATIONS (Cont.)
|
In addition, on January 6, 2022, the Company and certain former Trichome directors, one of which is currently serving as chairman of the Company's board of directors, signed an amendment to the tax indemnification agreement, and agreed to indemnify and hold harmless the Company and pay the Company the following amounts in cash as soon as practicable and in no event no later than February 28, 2022:
|
NOTE 5:-
|
BUSINESS COMBINATIONS (Cont.)
|
|
Proforma results
for the year ended December 31, 2021
|
||||
|
Revenues
|
$
|
|||
|
Net loss
|
$
|
( |
) |
These proforma results are based on estimates and assumptions, which the Company believes are reasonable. They are not necessarily the results that would have been realized had the Company and TFC been a combined company during the period presented and are not necessarily indicative of the Company's consolidated results of operations in future periods. The proforma results include adjustments related to purchase accounting, primarily amortization of intangible assets, depreciation related to the excess of fair value over cost attributable to purchased property, plant and equipment and elimination of inter-company transactions.
|
NOTE 5:-
|
BUSINESS COMBINATIONS (Cont.)
|
|
Proforma results for the year ended December
31, 2021
|
||||
|
Revenues
|
$ |
|
||
|
Net loss
|
$
|
(
|
) |
|
|
NOTE 5:-
|
BUSINESS COMBINATIONS (Cont.)
|
|
NOTE 5:-
|
BUSINESS COMBINATIONS (Cont.)
|
The fair value of the identifiable assets acquired and liabilities assumed on the acquisition date based on a final adjusted valuation performed in 2022, are as follows:
|
Preliminary PPA
|
Adjustments
|
Final PPA
|
||||||||||
|
Inventory
|
$
|
|
$
|
|
$
|
|
||||||
|
Advance payment for intangible assets of pharmacy
|
|
|
|
|||||||||
|
Property, plant and equipment
|
|
|
|
|||||||||
|
Intangible assets
|
|
(
|
)
|
|
||||||||
|
Total identifiable assets
|
|
|
|
|||||||||
|
Goodwill arising on acquisition
|
|
(
|
)
|
|
||||||||
|
Total purchase price
|
$
|
|
$
|
|
$
|
|
||||||
|
NOTE 5:-
|
BUSINESS COMBINATIONS (Cont.)
|
|
Proforma results for the year
ended December 31, 2021
|
||||
|
Revenues
|
$
|
|
||
|
Net loss
|
$
|
(
|
)
|
|
|
NOTE 5:-
|
BUSINESS COMBINATIONS (Cont.)
|
|
Proforma results
for the year
ended December
31, 2021
|
||||
|
Revenues
|
$
|
|
||
|
Net loss
|
$
|
(
|
)
|
|
|
NOTE 5:-
|
BUSINESS COMBINATIONS (Cont.)
|
|
Proforma results
for the year
ended December 31, 2021
|
||||
|
Revenues
|
$
|
|
||
|
Net loss
|
$
|
(
|
)
|
|
|
NOTE 5:-
|
BUSINESS COMBINATIONS (Cont.)
|
|
Fair value
|
||||||||||||||||||||||||
|
TFC
|
MYM
|
Vironna
|
Pharm Yarok
|
Oranim
|
Panaxia
|
|||||||||||||||||||
|
Assets |
||||||||||||||||||||||||
|
Cash and cash equivalents |
$ |
$ |
$ |
$ |
$ |
$ |
||||||||||||||||||
|
Trade and other receivables
|
|
|
|
|
|
|
||||||||||||||||||
|
Indemnification asset
|
|
|
|
|
|
|
||||||||||||||||||
|
Biological assets
|
|
|
|
|
|
|
||||||||||||||||||
|
Inventory
|
|
|
|
|
|
|
||||||||||||||||||
|
Loan receivable
|
|
|
|
|
|
|
||||||||||||||||||
|
Investments
|
|
|
|
|
|
|
||||||||||||||||||
|
Property, plant and equipment
|
|
|
|
|
|
|
||||||||||||||||||
|
Derivative assets
|
|
|
|
|
|
|
||||||||||||||||||
|
Right of use assets
|
|
|
|
|
|
|
||||||||||||||||||
|
Investments
|
|
|
|
|
|
|
||||||||||||||||||
|
Intangible assets
|
|
|
|
|
|
|
||||||||||||||||||
|
Total identifiable assets
|
|
|
|
|
|
|
||||||||||||||||||
|
Liabilities
|
||||||||||||||||||||||||
|
Trade and other payables
|
(
|
)
|
(
|
)
|
(
|
)
|
(
|
)
|
(
|
)
|
|
|||||||||||||
|
Bank loans
|
|
(
|
)
|
|
|
|
|
|||||||||||||||||
|
Lease liability
|
(
|
)
|
(
|
)
|
|
|
(
|
)
|
|
|||||||||||||||
|
Long term loans
|
|
|
|
(
|
)
|
|
|
|||||||||||||||||
|
Deferred tax, net
|
|
(
|
)
|
(
|
)
|
(
|
)
|
(
|
)
|
|
||||||||||||||
|
Total identifiable liabilities
|
(
|
)
|
(
|
)
|
(
|
)
|
(
|
)
|
(
|
)
|
|
|||||||||||||
|
Total identifiable assets, net
|
|
|
|
|
|
|
||||||||||||||||||
|
Goodwill arising on acquisition
|
|
|
|
|
|
|
||||||||||||||||||
|
Non-controlling interest
|
|
|
(
|
)
|
|
(
|
)
|
|
||||||||||||||||
|
Total purchase price
|
$
|
|
$
|
|
$
|
|
$
|
|
$
|
|
$
|
|
||||||||||||
| NOTE 6:- |
TRADE RECEIVABLES
|
| NOTE 7:- |
OTHER ACCOUNTS RECEIVABLE
|
|
December 31,
|
||||||||
|
2022
|
2021
|
|||||||
|
Prepaid expenses
|
$
|
|
$
|
|
||||
|
Government authorities
|
|
|
||||||
|
Related parties (see Note 21)
|
|
|
||||||
|
Indemnification assets (see Note 5)
|
|
|
||||||
|
Other receivables
|
|
|
||||||
|
$
|
|
$
|
|
|||||
| NOTE 8:- |
BIOLOGICAL ASSETS
|
|
Balance at of January 1, 2021
|
$
|
|
||
|
Additions related to acquisitions of Trichome and MYM
|
|
|||
|
Production costs capitalized
|
|
|||
|
Changes in fair value less cost to sell due to biological transformation
|
|
|||
|
Transferred to inventory upon harvest
|
(
|
)
|
||
|
Foreign exchange translation
|
|
|||
|
Balance at of December 31, 2021
|
|
|||
|
Production costs capitalized
|
|
|||
|
Changes in fair value less cost to sell due to biological transformation
|
|
|||
|
Transferred to inventory upon harvest
|
(
|
)
|
||
|
Restructuring disposal
|
( |
) | ||
|
Foreign exchange translation
|
|
|
||
|
Deconsolidation of Trichome (see Note 24)
|
(
|
)
|
||
|
Balance at of December 31, 2022
|
$
|
|
| NOTE 8:- |
BIOLOGICAL ASSETS (Cont.)
|
| 1. |
Selling price per gram - calculated as the weighted average historical selling price for all strains of cannabis sold by the Group, which is expected to approximate future selling prices.
|
| 2. |
Post-harvest costs - calculated as the cost per gram of harvested cannabis to complete the sale of cannabis plants post-harvest, consisting of the cost of direct and indirect materials, depreciation and labor as well as labelling and packaging costs.
|
| 3. |
Attrition rate - represents the weighted average percentage of biological assets which are expected to fail to mature into cannabis plants that can be harvested.
|
| 4. |
Average yield per plant - represents the expected number of grams of finished cannabis inventory which are expected to be obtained from each harvested cannabis plant.
|
| 5. |
Stage of growth - represents the weighted average number of weeks out of the average weeks growing cycle that biological assets have reached as of the measurement date. The growing cycle is approximately 12 weeks.
|
|
December 31,
|
10% change as at
December 31,
|
|||||||||||||||
|
2022
|
2021
|
2022
|
2021
|
|||||||||||||
|
Average selling price per gram of dried cannabis (in CAD)
|
$
|
|
$
|
|
$
|
|
$
|
|
||||||||
|
Average post-harvest costs per gram of dried cannabis (in CAD)
|
$
|
|
$
|
|
$
|
|
$
|
|
||||||||
|
Attrition rate
|
|
%
|
|
%
|
|
%
|
|
%
|
||||||||
|
Average yield per plant (in grams)
|
|
|
|
|
||||||||||||
|
Average stage of growth
|
|
%
|
|
%
|
|
%
|
|
%
|
||||||||
| NOTE 9:- |
INVENTORY
|
|
December 31, 2022
|
||||||||||||
|
Capitalized
costs
|
Fair valuation
adjustment, net
|
Carrying
value
|
||||||||||
|
Work in progress:
|
||||||||||||
|
Bulk cannabis
|
$
|
|
$
|
|
$
|
|
||||||
|
Finished goods:
|
||||||||||||
|
Packaged dried cannabis
|
|
$
|
|
|
||||||||
|
Other products
|
|
|
|
|||||||||
|
Balance as of December 31, 2022
|
$
|
|
$
|
|
$
|
|
||||||
|
December 31, 2021
|
||||||||||||
|
Capitalized
costs
|
Fair valuation
adjustment, net
|
Carrying
value
|
||||||||||
|
Work in progress:
|
||||||||||||
|
Bulk cannabis
|
$
|
|
$
|
|
$
|
|
||||||
|
Other cannabis products
|
|
|
|
|||||||||
|
Finished goods:
|
||||||||||||
|
Packaged dried cannabis
|
|
|
|
|||||||||
|
Other cannabis products
|
|
|
|
|||||||||
|
Other products
|
|
|
|
|||||||||
|
Balance as of December 31, 2021
|
$
|
|
$
|
|
$
|
|
||||||
| NOTE 10:- |
PROPERTY, PLANT AND EQUIPMENT, NET
|
|
Buildings and
improvements
|
Production
equipment and
furniture
|
Greenhouse
structure
|
Computer,
software and
equipment
|
Motor
vehicles
|
Total
|
|||||||||||||||||||
|
Cost:
|
||||||||||||||||||||||||
|
Balance at January 1, 2021
|
$
|
|
$
|
|
$
|
|
$
|
|
$
|
|
$
|
|
||||||||||||
|
Additions during the year
|
|
|
|
|
|
|
||||||||||||||||||
|
Additions related to acquisitions
|
|
|
|
|
|
|
||||||||||||||||||
|
Foreign currency translation
|
(
|
)
|
|
|
|
|
|
|||||||||||||||||
|
Balance at December 31, 2021
|
|
|
|
|
|
|
||||||||||||||||||
|
Additions during the year
|
|
|
|
|
|
|
||||||||||||||||||
|
Deconsolidation of Trichome
|
(
|
)
|
(
|
)
|
|
(
|
)
|
(
|
)
|
(
|
)
|
|||||||||||||
|
Foreign currency translation
|
(
|
)
|
(
|
)
|
(
|
)
|
(
|
)
|
(
|
)
|
(
|
)
|
||||||||||||
|
Balance at December 31, 2022
|
|
|
|
|
|
|
||||||||||||||||||
|
Accumulated depreciation:
|
||||||||||||||||||||||||
|
Balance at January 1, 2021
|
|
|
|
|
|
|
||||||||||||||||||
|
Depreciation during the year
|
|
|
|
|
|
|
||||||||||||||||||
|
Foreign currency translation
|
|
|
|
|
|
|
||||||||||||||||||
|
Balance at December 31, 2021
|
|
|
|
|
|
|
||||||||||||||||||
|
Depreciation during the year
|
|
|
|
|
|
|
||||||||||||||||||
|
Impairment
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||
|
Deconsolidation of Trichome
|
(
|
)
|
(
|
)
|
|
(
|
)
|
(
|
)
|
(
|
)
|
|||||||||||||
|
Foreign currency translation
|
(
|
)
|
(
|
)
|
(
|
)
|
(
|
)
|
(
|
)
|
(
|
)
|
||||||||||||
|
Balance at December 31, 2022
|
|
|
|
|
|
|
||||||||||||||||||
|
Depreciated cost at December 31, 2022
|
$
|
|
$
|
|
$
|
|
$
|
|
$
|
|
$
|
|
||||||||||||
|
Depreciated cost at December 31, 2021
|
$
|
|
$
|
|
$
|
|
$
|
|
$
|
|
$
|
|
||||||||||||
| NOTE 11:- |
GOODWILL AND INTANGIBLE ASSETS, NET
|
|
Cultivations
and processing license *) |
Customer
relationships |
Brand
|
Goodwill
|
Other
|
Total
|
|||||||||||||||||||
|
Cost:
|
||||||||||||||||||||||||
|
Balance at January 1, 2021
|
$
|
|
$
|
|
$
|
|
$
|
|
$
|
|
$
|
|
||||||||||||
|
Initial consolidation
|
|
|
|
|
|
|
||||||||||||||||||
|
Foreign currency translation adjustments
|
(
|
)
|
|
|
|
|
|
|||||||||||||||||
|
Balance at December 31, 2021
|
|
|
|
|
|
|
||||||||||||||||||
|
PPA adjustments during measurement period
|
|
|
|
(
|
)
|
|
|
|||||||||||||||||
|
Disposals
|
(
|
)
|
|
|
|
|
(
|
)
|
||||||||||||||||
|
Deconsolidation of Trichome
|
(
|
)
|
(
|
)
|
(
|
)
|
|
(
|
)
|
(
|
)
|
|||||||||||||
|
Foreign currency translation adjustments
|
|
(
|
)
|
|
(
|
)
|
(
|
)
|
(
|
)
|
||||||||||||||
|
Balance at December 31, 2022
|
|
|
|
|
|
|
||||||||||||||||||
|
Accumulated amortization:
|
||||||||||||||||||||||||
|
Balance at January 1, 2021
|
|
|
|
|
|
|
||||||||||||||||||
|
Amortization recognized in the year
|
|
|
|
|
|
|
||||||||||||||||||
|
Impairment
|
|
|
|
|
|
(
|
)
|
|||||||||||||||||
|
Balance at December 31, 2021
|
|
|
|
|
|
|
||||||||||||||||||
|
Amortization recognized in the year
|
|
|
|
|
|
|
||||||||||||||||||
|
Impairment
|
|
|
|
|
|
|
|
|||||||||||||||||
|
Deconsolidation of Trichome
|
(
|
)
|
(
|
)
|
|
|
(
|
)
|
(
|
)
|
||||||||||||||
|
Balance at December 31, 2022
|
|
|
|
|
|
|
||||||||||||||||||
|
Amortized cost at December 31, 2022
|
|
|
|
|
|
|
||||||||||||||||||
|
Amortized cost at December 31, 2021
|
$
|
|
$
|
|
$
|
|
$
|
|
$
|
|
$
|
|
||||||||||||
| *) |
The licenses consist of GMP and GDP licenses.
|
| NOTE 11:- |
INTANGIBLE ASSETS, NET (Cont.)
|
| NOTE 12:- |
RIGHT-OF-USE ASSETS
|
|
Land and
buildings
|
Motor
vehicles
|
Total
|
||||||||||
|
Cost:
|
||||||||||||
|
Balance at January 1, 2021
|
$
|
|
$
|
|
$
|
|
||||||
|
Additions during the year:
|
||||||||||||
|
New leases
|
|
|
|
|||||||||
|
Additions related to business combinations
|
|
|
|
|||||||||
|
Currency translation adjustments
|
|
|
|
|||||||||
|
Balance at December 31, 2021
|
|
|
|
|||||||||
|
Additions during the year:
|
||||||||||||
|
New leases
|
|
|
|
|||||||||
|
Disposals during the year
|
(
|
)
|
|
(
|
)
|
|||||||
|
Termination of leases
|
(
|
)
|
(
|
)
|
(
|
)
|
||||||
|
Deconsolidation of Trichome
|
(
|
)
|
(
|
)
|
(
|
)
|
||||||
|
Currency translation adjustments
|
(
|
)
|
(
|
)
|
(
|
)
|
||||||
|
Balance at December 31, 2022
|
|
|
|
|||||||||
|
Accumulated depreciation:
|
||||||||||||
|
Balance at January 1, 2021
|
|
|
|
|||||||||
|
Additions during the year:
|
||||||||||||
|
Depreciation and amortization
|
|
|
|
|||||||||
|
Currency translation adjustments
|
|
|
|
|||||||||
|
Balance at December 31, 2021
|
|
|
|
|||||||||
|
Additions during the year:
|
||||||||||||
|
Depreciation and amortization
|
|
|
|
|||||||||
|
Termination of leases
|
(
|
)
|
(
|
)
|
(
|
)
|
||||||
|
Impairment |
|
|
||||||||||
|
Deconsolidation of Trichome
|
(
|
)
|
(
|
)
|
(
|
)
|
||||||
|
Currency translation adjustments
|
(
|
)
|
(
|
)
|
(
|
)
|
||||||
|
Balance at December 31, 2022
|
$
|
|
$
|
|
$
|
|
||||||
|
Depreciated cost at December 31, 2022
|
$
|
|
$
|
|
$
|
|
||||||
|
Depreciated cost at December 31, 2021
|
$
|
|
$
|
|
$
|
|
||||||
| NOTE 13:- |
EMPLOYEE BENEFIT ASSETS AND LIABILITIES
|
|
a.
|
Defined benefit plans:
|
| b. |
Expenses recognized in the consolidated statements of profit or loss and other comprehensive income:
|
|
Year ended
December 31,
|
||||||||
|
2022
|
2021
|
|||||||
|
Current service cost
|
$
|
|
$
|
|
||||
|
Interest expenses
|
|
|
||||||
|
Total employee benefit expenses
|
$
|
|
$
|
|
||||
|
Interest income on plan assets
|
$
|
|
$
|
|
||||
| c. |
The defined benefit liability, net:
|
|
December 31,
|
||||||||
|
2022
|
2021
|
|||||||
|
Defined benefit obligation
|
$
|
|
$
|
|
||||
|
Fair value of plan assets
|
(
|
)
|
(
|
)
|
||||
|
Net defined benefit liability
|
$
|
|
$
|
|
||||
| NOTE 13:- |
EMPLOYEE BENEFIT ASSETS AND LIABILITIES (Cont.)
|
| d. |
Changes in the present value of defined benefit liabilities:
|
|
2022
|
2021
|
|||||||
|
Balance at January 1,
|
$
|
|
$
|
|
||||
|
Current service cost
|
|
|
||||||
|
Interest expenses
|
|
|
||||||
|
Benefits paid
|
(
|
)
|
(
|
)
|
||||
|
Re-measurement loss on defined benefit plans
|
(
|
)
|
(
|
)
|
||||
|
Foreign currency translation effect
|
(
|
)
|
|
|||||
|
Balance at December 31,
|
$
|
|
$
|
|
||||
| e. |
Changes in the fair value of plan assets:
|
|
2022
|
2021
|
|||||||
|
Balance at January 1,
|
$
|
|
$
|
|
||||
|
Interest income
|
|
|
||||||
|
Return, net of interest income - remeasurement gain (loss)
|
|
(
|
)
|
|||||
|
Benefits paid
|
(
|
)
|
(
|
)
|
||||
|
Amounts deposited
|
|
|
||||||
|
Foreign currency translation effect
|
|
(
|
)
|
|||||
|
Balance at December 31,
|
$
|
|
$
|
|
||||
| f. |
The principal assumptions underlying the defined benefit plan:
|
|
2022
|
2021
|
|||||||
|
%
|
||||||||
|
Discount rate
|
|
|
||||||
|
Salary growth
|
|
|
||||||
| NOTE 14:- |
OTHER PAYABLES
|
|
December 31,
|
||||||||
|
2022
|
2021
|
|||||||
|
Accrued expenses
|
$
|
|
$
|
|
||||
|
Employees and payroll accruals
|
|
|
||||||
|
Government authorities
|
|
|
||||||
|
Related parties
|
|
|
||||||
|
Advances from customers
|
|
|
||||||
|
Other payables - restructuring
|
|
|
||||||
|
Other payables
|
|
|
||||||
|
$
|
|
$
|
|
|||||
|
NOTE 15:-
|
FINANCIAL INSTRUMENTS
|
|
December 31,
|
|||||||||||
|
Note
|
2022
|
2021
|
|||||||||
|
Financial assets:
|
|||||||||||
|
Investments
|
c,e
|
|
$
|
|
$
|
|
|||||
|
Derivative assets
|
$
|
|
$
|
|
|||||||
|
Financial liabilities:
|
|||||||||||
|
Warrants
|
b,d
|
|
$
|
(
|
)
|
$
|
(
|
)
|
|||
| a. |
Management believes that the carrying amount of cash and cash equivalents, trade receivables, other accounts receivable, loans receivables, trade payables, bank loans, other account payables and accrued expenses and purchase consideration payable, and approximate their fair value due to the short-term maturities of these instruments.
|
| b. |
For the years ended December 31, 2022 and 2021, the Company recognized a revaluation gain (loss) from remeasurement of Warrants of $
|
| NOTE 15:- |
FINANCIAL INSTRUMENTS (Cont.)
|
| c. |
On December 26, 2019, IMC entered into a share purchase agreement (the “SPA”) with Xinteza API Ltd. ("Xinteza"), a company with a unique biosynthesis technology.
|
| d. |
On May 10, 2021, the Company completed an overnight marketed offering (the “Offering”) of
|
| NOTE 15:- |
FINANCIAL INSTRUMENTS (Cont.)
|
|
December 31,
|
December 31,
|
|||||||||
|
2022
|
2021
|
Sensitivity
|
||||||||
|
Expected volatility
|
|
|
Increase (decrease) in key assumptions would result in increase (decrease) in fair value
|
|||||||
|
Expected life (in years)
|
|
|
||||||||
|
Risk-free interest rate
|
|
|
Increase (decrease) in key assumptions would result in decrease (increase) in fair value
|
|||||||
|
Expected dividend yield
|
|
|
||||||||
|
Fair value:
|
||||||||||
|
Per Warrant (Canadian Dollar)
|
$
|
$
|
||||||||
|
Total Warrants (Canadian Dollar in thousands)
|
$
|
$
|
||||||||
| e. |
Financial risk management:
|
| NOTE 15:- |
FINANCIAL INSTRUMENTS (Cont.)
|
|
Less than one year
|
1 to 5 years
|
6 to 10
Years
|
>10
years
|
|||||||||||||
|
Lease liabilities
|
$
|
|
$
|
|
$
|
|
$
|
|
||||||||
|
Bank loans and others
|
$
|
|
$
|
|
$
|
|
$
|
|
||||||||
|
Total
|
$
|
|
$
|
|
$
|
|
$
|
|
||||||||
| NOTE 15:- |
FINANCIAL INSTRUMENTS (Cont.)
|
|
Less than one year
|
1 to 5 years
|
6 to 10
Years
|
>10
years
|
|||||||||||||
|
Lease liabilities
|
$
|
|
$
|
|
$
|
|
$
|
|
||||||||
|
Bank loans
|
|
|
|
|
||||||||||||
|
Total
|
$
|
|
$
|
|
$
|
|
$
|
|
||||||||
The maturity profile of the Group's other financial liabilities with liquidity risk (trade payables, other account payable and accrued expenses) as of December 31, 2022 and 2021, are less than one year.
| NOTE 15:- |
FINANCIAL INSTRUMENTS (Cont.)
|
| f. |
Changes in liabilities arising from financing activities:
|
|
Loans
|
Lease
liabilities |
Warrants
|
Total
liabilities arising from financing activities |
|||||||||||||
|
Balance as of January 1, 2021
|
$
|
|
$
|
|
$
|
|
$
|
|
||||||||
|
Issuance of new warrants
|
|
|
|
|
||||||||||||
|
Additions for new loans
|
|
|
|
|
||||||||||||
|
Additions for new leases
|
|
|
|
|
||||||||||||
|
Additions related to acquisitions
|
|
|
|
|
||||||||||||
|
Repayments
|
(
|
)
|
(
|
)
|
|
(
|
)
|
|||||||||
|
Effective interest
|
|
|
|
|
||||||||||||
|
Other changes
|
|
|
(
|
)
|
(
|
)
|
||||||||||
|
Effect of changes in fair value
|
|
|
(
|
)
|
(
|
)
|
||||||||||
|
Balance as of December 31, 2021
|
|
|
|
|
||||||||||||
|
Additions for new loans
|
|
|
|
|
||||||||||||
|
Additions for new leases
|
|
|
|
|
||||||||||||
|
Repayments
|
|
(
|
)
|
|
(
|
)
|
||||||||||
|
Effective interest
|
|
|
|
|
||||||||||||
|
Effect of exchange rate differences
|
(
|
)
|
(
|
)
|
|
(
|
)
|
|||||||||
|
Deconsolidation of Trichome
|
(
|
)
|
(
|
)
|
|
(
|
)
|
|||||||||
|
Effect of changes in fair value
|
|
|
(
|
)
|
(
|
)
|
||||||||||
|
Balance as of December 31, 2022
|
$
|
|
$
|
|
$
|
|
$
|
|
||||||||
| NOTE 16:- |
CONTINGENT LIABILITIES, GUARANTEES, COMMITMENTS AND CHARGES
|
| a. |
On August 19, 2019, a cannabis consumer (the “Applicant”) filed a motion for approval of a class action to Tel Aviv - Jaffa District Court (the “Motion”) against 17 companies (the “Parties”) operating in the field of medical cannabis in Israel, including Focus. The Applicant’s argument is that the Parties did not accurately mark the concentration of active ingredients in their products. The personal suit sum for each class member stands at NIS
Due to the current preliminary state of the litigation process and based on the opinion of legal counsel to Focus, the Company’s management believes that it is not reasonably possible to assess the outcome of the proceeding. Therefore, no provision has been recorded in respect thereof.
|
| b. |
On July 11, 2021 the Company was informed that on June 30, 2021, a claim was filed to Beer Sheva Magistrate Court, by the municipal committee presiding over planning and construction in southern Israel against Focus, Focus’ directors and officers, including Oren Shuster and Rafael Gabay, and certain landowners, claiming for inadequate permitting for construction relating to the Focus Facility (the “Construction Proceedings”).
On December 6, 2021 the defendants filed a motion request for dismissal the indictment on the ground of defense of justice. The municipal committee filed its response and after that the defendants filed a response to the municipal committee’s response. As of the date of this letter no decision has yet been made on the application.
|
| NOTE 16:- |
CONTINGENT LIABILITIES, GUARANTEES, COMMITMENTS AND CHARGES (Cont.)
|
|
A hearing was initially set to December 1, 2021 but postponed several times in order to allow the parties to negotiate towards a resolution. The hearing is set June 22, 2023. A draft agreement between the parties sent by the defendant to the municipal committee in order for it to be sent to the state attorney’s office for their comments, which once obtained, will be filed with the Court for its approval. The Court is not obligated to approve the agreement between the parties, if obtained.
At this preliminary stage, based on the opinion of its legal counsel, Focus’ management cannot assess the chances of the claim advancing or the potential outcome of the Construction Proceedings.
|
| c. |
On November 19, 2021, Adjupharm filed a statement of claim (the “Claim”) to the District Court of Stuttgart (the “Stuttgart Court”) against Stroakmont & Atton Trading GmbH (“Stroakmont & Atton”), its shareholders and managing directors regarding a debt owed by Stroakmont & Atton to Adjupharm in an amount of approximately EUR
|
| 1. |
that the contractual partner of the Company is not the defendant, Stroakmont & Atton is not the real purchaser rather a company named Uniclaro GmbH.
|
| 2. |
that the Company allegedly placed an order with Uniclaro GmbH for a total of
|
|
On March 22, 2022 Adjupharm filed a response to Stroakmont & Atton’s statement of defence and rejected both allegations with a variety of legal arguments and facts and also offered evidence to the contrary in the form of testimony from the witnesses in question.
The burden of proof for both allegations lies with the opponents and they offered evidences to the court in the form of testimony from certain witnesses. If the opponents succeed in proving both allegations to the court, the chances of winning the lawsuit will be considerably reduced. However, it will not be easy for the opponents to present evidence of these allegations.
On May 27, 2022, the conciliation hearing and main hearing were held. The Stuttgart Court ruled that the Company shall submit another writ by August 29, 2022. The Stuttgart Court also scheduled a pronouncement date for September 7, 2022, when the Stuttgart Court will enter a judgement or hold an evidentiary hearing with witnesses. Following the pronouncement date on September 7, 2022 an evidentiary hearing with witnesses was held on two occasions, January 11, 2023, where witnesses on behalf of Adjupharm testified, and on February 22, 2023, witnesses on behalf of Stroakmont & Atton testified.
The court provided the parties a deadline until March 24 2023 to evaluate the testimonies of the witnesses and to deliver to the court a summary of the factual and legal situation after the court hearings. The court will announce its decision for further proceedings or its judgment on April 5, 2023. At this stage, the Company management cannot assess the chances of the claim advancing or the potential outcome of this these proceedings.
|
| NOTE 17:- |
TAXES ON INCOME
|
| a. |
Tax rates applicable to the Group:
|
| 1. |
The Company is subject to tax rates applicable in Canada. The combined federal and provincial rate for 2022 and 2021 is
|
| 2. |
The Israeli subsidiaries are subject to Israeli corporate income tax rate of
|
| 3. |
The German subsidiary is subject to weighted tax rate of approximately
|
| b. |
Carryforward losses for tax purposes: |
| c. |
Income tax expense (benefit): |
|
Year ended
December 31,
|
||||||||||||
|
2022
|
2021
|
2020 | ||||||||||
|
Current
|
$
|
$
|
$ |
|||||||||
|
Deferred, net
|
( |
) |
|
( |
) | |||||||
|
Income tax from previous years
|
( |
)
|
( |
) | ||||||||
|
$
|
( |
) |
$
|
$ |
||||||||
| NOTE 17:- |
TAXES ON INCOME (Cont.)
|
|
d.
|
Deferred taxes:
|
|
Statements of
financial position
|
||||||||
|
December 31,
|
||||||||
|
2022
|
2021
|
|||||||
|
Deferred tax assets:
|
||||||||
|
Carryforward tax losses and other
|
$
|
|
$
|
|
||||
|
Other deferred tax assets
|
|
|
||||||
|
|
|
|||||||
|
Deferred tax liabilities:
|
||||||||
|
Inventory and biological assets
|
|
|
||||||
|
Intangible assets
|
|
|
||||||
|
Other
|
|
|
||||||
|
|
|
|||||||
|
Deferred tax liabilities, net
|
$
|
(
|
)
|
$
|
(
|
)
|
||
|
December 31,
|
||||||||
|
2022
|
2021
|
|||||||
|
Non-current assets
|
$
|
|
$
|
|
||||
|
Non-current liabilities
|
$
|
|
$
|
|
||||
| NOTE 17:- |
TAXES ON INCOME (Cont.)
|
| e. |
Reconciliation of tax expense (benefit) and the accounting loss multiplied by the Company's domestic tax rate for:
|
|
Year ended
December 31,
|
||||||||||||
|
2022
|
2021
|
2020
|
||||||||||
|
Loss before income tax
|
$
|
(
|
)
|
$
|
(
|
)
|
$
|
(
|
)
|
|||
|
Statutory tax rate in Canada
|
(
|
)
|
(
|
)
|
(
|
)
|
||||||
|
Increase (decrease) in income tax due to:
|
||||||||||||
|
Non-deductible expenses (non-taxable income), net for tax purposes
|
|
(
|
)
|
|
||||||||
|
Effect of different tax rate of subsidiaries
|
|
|
|
|||||||||
|
Adjustments in respect of current income tax of previous years
|
(
|
)
|
(
|
)
|
|
|||||||
|
Recognition (derecognition) of tax benefit in respect of losses of previous years
|
|
|
(
|
)
|
||||||||
|
Unrecognized tax benefit in respect of loss for the year
|
|
|
|
|||||||||
|
|
|
|
|
|
||||||||
|
Other adjustments
|
(
|
)
|
(
|
)
|
|
|||||||
|
Income tax expense (benefit)
|
$
|
(
|
)
|
$
|
|
$
|
|
|||||
| NOTE 18:- |
EQUITY
|
| a. |
Composition of share capital:
|
|
December 31, 2022
|
December 31, 2021
|
|||||||||||||||
|
Authorized
|
Issued and outstanding
|
Authorized
|
Issued and outstanding
|
|||||||||||||
|
Number of shares
|
||||||||||||||||
|
Common Shares without par value
|
Unlimited | Unlimited | ||||||||||||||
| b. |
Capital issuances:
|
| NOTE 18:- |
EQUITY (Cont.)
|
On March 14, 2022, the Pharm Yarok Transaction closed upon receipt of all requisite approvals, including the IMCA approval. In connection with closing of the Pharm Yarok Transaction, the Company completed a non-brokered private placement with former shareholders of Pharm Yarok and Rosen High Way on March 14, 2022. A total of
On March 14, 2022, the Vironna Transaction closed upon receipt of all requisite approvals, including the approval of the IMCA and NIS
On March 28, 2022, the Oranim Transaction closed upon receipt of all requisite approvals, including the approval of the MOH and NIS
On August 19, 2022, the Company announced a private placement for aggregate gross proceeds of up to $
| c. |
Changes in issued and outstanding share capital:
|
|
Number of shares
|
||||
|
Balance as of January 1, 2021
|
|
|||
|
Common Shares issued as a result of Warrants and Compensation options exercised
|
|
|||
|
Common Shares issued as a result of options exercises
|
|
|||
|
Purchase of treasury common shares
|
(
|
)
|
||
|
Common Shares issued related secondary transaction and business combinations
|
|
|||
|
Balance as of December 31, 2021
|
|
|||
|
Common Shares issued as a result of options exercises
|
|
|||
|
Common shares issued in settlement of purchase consideration of business combination
|
|
|||
|
Issuance of treasury common shares
|
|
|||
|
Issuance of Common Shares
|
|
|||
|
Balance as of December 31, 2022
|
|
|||
| NOTE 18:- |
EQUITY (Cont.)
|
| d. |
Share option plan:
|
|
Year ended
December 31,
|
||||||||
|
2022 |
2021 |
|||||||
|
Exercise price (in CAD)
|
$
|
$
|
||||||
|
Dividend yield (%)
|
|
|
||||||
|
Expected life of share options (Years)
|
|
|
||||||
|
Volatility (%)
|
|
|
||||||
|
Annual risk-free rate (%)
|
|
|
||||||
|
Share price (in CAD)
|
$
|
$
|
||||||
| NOTE 18:- |
EQUITY (Cont.)
|
|
Year ended December 31, 2022
|
||||||||
|
Number of
options |
Weighted
average exercise price |
|||||||
|
in CAD
|
||||||||
|
Options outstanding at the beginning of the year
|
||||||||
|
Options granted during the year
|
||||||||
|
Options exercised during the year
|
( |
)
|
||||||
|
Options forfeited during the year
|
( |
)
|
||||||
|
Options outstanding at the end of year
|
$ | |||||||
|
Options exercisable at the end of year
|
$ | |||||||
* Includes
|
Year ended December 31, 2021
|
||||||||
|
Number of
options |
Weighted
average exercise price |
|||||||
|
in CAD
|
||||||||
|
Options outstanding at the beginning of the year
|
||||||||
|
Options granted during the year
|
||||||||
|
Options exercised during the year
|
( |
)
|
||||||
|
Options forfeited during the year
|
( |
)
|
||||||
|
Options outstanding at the end of year
|
$ | |||||||
|
Options exercisable at the end of year
|
$ | |||||||
| NOTE 18:- |
EQUITY (Cont.)
|
|
Number of RSU
|
||||
|
Outstanding at the beginning of the year
|
|
|||
|
Granted during the year
|
|
|||
|
Outstanding at the end of the year
|
|
|||
|
Exercisable at the end of year
|
|
|||
| e. |
Other convertible securities:
|
| NOTE 19:- |
SELECTED STATEMENTS OF PROFIT OR LOSS DATA
|
|
Year ended
December 31,
|
||||||||
|
2022
|
2021
|
|||||||
|
Salaries and related expenses
|
$
|
|
$
|
|
||||
|
Depreciation and amortization
|
$
|
|
$
|
|
||||
| NOTE 20:- |
NET LOSS PER SHARE
|
|
Year ended December 31,
|
||||||||||||||||
|
2022
|
2021
|
|||||||||||||||
|
Weighted number of shares (in thousands)
|
Net loss attributable to equity holders of the Company
|
Weighted number of shares (in thousands)
|
Net loss attributable to equity holders of the Company
|
|||||||||||||
|
For the computation of basic net earnings from continuing operations
|
|
$
|
(
|
)
|
|
$
|
(
|
)
|
||||||||
|
Effect of potential dilutive Ordinary shares - Warrants
|
|
(
|
)
|
|
(
|
)
|
||||||||||
|
For the computation of diluted net earnings from continuing operations (*)
|
|
$
|
(
|
)
|
|
$
|
(
|
)
|
||||||||
|
For the computation of basic and diluted net earnings from discontinued operations (*)
|
|
$
|
(
|
)
|
|
$
|
(
|
)
|
||||||||
| *) |
For 2022 and 2021, potentially dilutive securities (share options) were excluded from the calculation of diluted earnings per share as they are antidilutive.
|
| **) |
Including the effect of Share Consolidation (See Note 18a).
|
| NOTE 21:- |
RELATED PARTY BALANCES AND TRANSACTIONS
|
| a. |
Balances and transactions:
|
|
December 31,
|
||||||||
|
2022
|
2021
|
|||||||
|
Other accounts receivables
|
$
|
|
$
|
|
||||
|
Other accounts payables
|
$
|
|
$
|
|
||||
| NOTE 21:- |
RELATED PARTY BALANCES AND TRANSACTIONS (Cont.)
|
|
Year ended
December 31,
|
||||||||
|
2022
|
2021
|
|||||||
|
General and administrative expenses
|
$
|
|
$
|
|
||||
| a. |
Compensation of key management personnel of the Group:
|
|
Year ended
December 31,
|
||||||||
|
2022
|
2021
|
|||||||
|
Payroll and related expenses
|
$
|
|
$
|
|
||||
|
Share-based compensation
|
$
|
|
$
|
|
||||
|
Professional fees *)
|
$
|
|
$
|
|
||||
| *) |
|
NOTE 22:- SUMMARIZED FINANCIAL INFORMATION FOR PARTLY OWNED SUBSIDIARY
|
December 31,
|
||||||||
|
2022
|
2021
|
|||||||
|
Statement of financial position at reporting date (as presented in Focus' financial statements):
|
||||||||
|
Current assets
|
$
|
|
$
|
|
||||
|
Non-current assets
|
|
|
||||||
|
Current liabilities
|
(
|
)
|
(
|
)
|
||||
|
Non-current liabilities
|
(
|
)
|
(
|
)
|
||||
|
Total equity (deficiency)
|
$
|
(
|
)
|
$
|
|
|||
|
Year ended
December 31,
|
||||||||
|
2022
|
2021
|
|||||||
|
Operating results (as presented in Focus' financial statements):
|
||||||||
|
Revenues
|
$
|
|
$
|
|
||||
|
Net loss
|
(
|
)
|
(
|
)
|
||||
|
Other comprehensive income
|
|
|
||||||
|
Total comprehensive loss
|
$
|
(
|
)
|
$
|
(
|
)
|
||
|
Year ended
December 31,
|
||||||||
|
2022
|
2021
|
|||||||
|
Cash flows (as presented in Focus' financial statements):
|
||||||||
|
From operating activities
|
$
|
(
|
)
|
$
|
|
|||
|
From investing activities
|
|
(
|
)
|
|||||
|
From financing activities
|
|
|
||||||
|
Effect of foreign exchange on cash and cash equivalents
|
(
|
)
|
|
|||||
|
Net increase (decrease) in cash and cash equivalents
|
$
|
(
|
)
|
$
|
|
|||
| NOTE 23:- |
OPERATING SEGMENTS
|
|
Israel
|
Germany
|
Adjustments
|
Total
|
|||||||||||||
|
Year ended December 31, 2022
|
||||||||||||||||
|
Revenue
|
$
|
|
$
|
|
$
|
|
$
|
|
||||||||
|
Segment loss
|
$
|
(
|
)
|
$
|
(
|
)
|
$
|
|
$
|
(
|
)
|
|||||
|
Unallocated corporate expenses
|
$
|
(
|
)
|
$
|
(
|
)
|
||||||||||
|
Total operating loss
|
$
|
(
|
)
|
|||||||||||||
|
Depreciation, amortization and impairment
|
$
|
|
$
|
|
$
|
|
$
|
|
||||||||
|
Israel
|
Germany
|
Adjustments
|
Total
|
|||||||||||||
|
Year ended December 31, 2021
|
||||||||||||||||
|
Revenue
|
$
|
|
$
|
|
$
|
|
$
|
|
||||||||
|
Segment loss
|
$
|
(
|
)
|
$
|
(
|
)
|
$
|
|
$
|
(
|
)
|
|||||
|
Unallocated corporate expenses
|
$
|
(
|
)
|
$
|
(
|
)
|
||||||||||
|
Total operating loss
|
$
|
(
|
)
|
|||||||||||||
|
Depreciation, amortization and impairment
|
$
|
|
$
|
|
$
|
|
$
|
|
||||||||
| NOTE 23:- |
OPERATING SEGMENTS (Cont.)
|
|
Israel
|
Germany
|
Adjustments
|
Total
|
|||||||||||||
|
Year ended December 31, 2020
|
||||||||||||||||
|
Revenue
|
$
|
|
$
|
|
$
|
|
$
|
|
||||||||
|
Segment loss
|
$
|
(
|
)
|
$
|
(
|
)
|
$
|
|
$
|
(
|
)
|
|||||
|
Unallocated corporate expenses
|
$
|
(
|
)
|
$
|
(
|
)
|
||||||||||
|
Total operating loss
|
$
|
(
|
)
|
|||||||||||||
|
Depreciation, amortization and impairment
|
$
|
|
$
|
|
$
|
|
$
|
|
||||||||
| NOTE 24:- |
DISCONTINUED OPERATIONS AND DECONSOLIDATION OF TRICHOME
|
| NOTE 24:- |
DISCONTINUED OPERATIONS AND DECONSOLIDATION OF TRICHOME (Cont.)
|
|
November 6,
2022
|
December 31,
2021
|
|||||||
|
ASSETS
|
||||||||
|
Current assets:
|
||||||||
|
Cash and cash equivalents
|
$
|
|
$
|
|
||||
|
Trade receivables
|
|
|
||||||
|
Other accounts receivable
|
|
|
||||||
|
Loans receivable
|
|
|
||||||
|
Biological assets
|
|
|
||||||
|
Inventories
|
|
|
||||||
|
|
|
|||||||
|
Non-current assets:
|
||||||||
|
Property, plant and equipment, net
|
|
|
||||||
|
Derivative assets
|
|
|
||||||
|
Right-of-use assets, net
|
|
|
||||||
|
Intangible assets, net
|
|
|
||||||
|
Goodwill
|
|
|
||||||
|
|
|
|||||||
|
Total Assets
|
$
|
|
$
|
|
||||
|
November 6,
2022
|
December 31,
2021
|
|||||||
|
LIABILITIES
|
||||||||
|
Current liabilities:
|
||||||||
|
Trade payables
|
$
|
|
$
|
|
||||
|
Bank loans and credit facilities
|
|
|
||||||
|
Other accounts payable and accrued expenses
|
|
|
||||||
|
Current maturities of operating lease liabilities
|
|
|
||||||
|
|
|
|||||||
|
Non-current liabilities:
|
||||||||
|
Operating lease liabilities
|
|
|
||||||
|
Deferred tax liability, net
|
|
|
||||||
|
|
|
|||||||
|
Total liabilities
|
$
|
|
$
|
|
||||
| NOTE 24:- |
DISCONTINUED OPERATIONS AND DECONSOLIDATION OF TRICHOME (Cont.)
|
|
Period ended November 6,
|
Year ended December 31
|
|||||||
|
2022
|
2021
|
|||||||
|
Revenues
|
$
|
|
$
|
|
||||
|
Cost of revenues
|
|
|
||||||
|
Gross profit before fair value adjustments
|
|
|
||||||
|
Fair value adjustments:
|
||||||||
|
Unrealized change in fair value of biological assets
|
|
|
||||||
|
Realized fair value adjustments on inventory sold in the period
|
(
|
)
|
(
|
)
|
||||
|
Total fair value adjustments
|
(
|
)
|
|
|||||
|
Gross profit
|
|
|
||||||
|
General and administrative expenses
|
|
|
||||||
|
Impairment of goodwill, intangible assets, right-of-use assets and fixed assets
|
|
|
||||||
|
Selling and marketing expenses
|
|
|
||||||
|
Restructuring expenses
|
|
|
||||||
|
Share-based compensation
|
|
|
||||||
|
Total operating expenses
|
|
|
||||||
|
Operating loss
|
(
|
)
|
(
|
)
|
||||
|
Finance expenses, net
|
(
|
)
|
(
|
)
|
||||
|
Loss before income taxes
|
(
|
)
|
(
|
)
|
||||
|
Income tax expense (benefit)
|
(
|
)
|
|
|||||
|
Net loss from discontinued operations, net of tax
|
$
|
(
|
)
|
$
|
(
|
)
|
||
|
Period ended November 6, 2022
|
Year ended December 31, 2021
|
|||||||
|
Operating activities
|
$
|
(
|
)
|
$
|
(
|
)
|
||
|
Investing activities
|
$
|
(
|
)
|
$
|
(
|
)
|
||
|
Financing activities
|
$
|
(
|
)
|
$
|
|
|||
| NOTE 25:- |
SUBSEQUENT EVENTS
|
| a. |
LIFE Offering
In January and February of 2023, the Company issued an aggregate of issued
In addition, a non-independent director of the Company subscribed for an aggregate of
|
| b. |
Concurrent Offering
Concurrent with the LIFE Offering, the Company issued an aggregate of
All Units issued under the Concurrent Offering were subject to a statutory hold period of four months and one day in accordance with applicable Canadian securities laws.
|